Facile Controlled Synthesis of Pd-ZnO Nanostructures for Nitrite Detection
Abstract
:1. Introduction
2. Results and Discussion
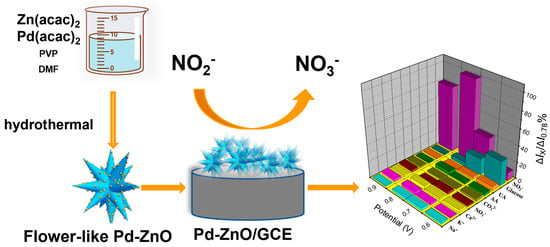
2.1. Synthesis and Characterization of Pd-ZnO Nanocatalysts
2.2. Electrocatalytic Properties of Pd-ZnO /GCE
2.3. Effects of Surface Structure
2.4. Effect of Scan Rate
2.5. Effect of the Solution pH
2.6. Electrochemical Detection of Nitrite
2.7. Reproducibility and Stability
2.8. Real Samples Analysis
3. Experimental Section
3.1. Chemical and Materials
3.2. Synthesis of Pd-ZnO Nanocomposites
3.3. Apparatus and Procedures
3.4. Electrochemical Measurements
3.5. Pretreatment of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Parvizishad, M.; Dalvand, A.; Mahvi, A.H.; Goodarzi, F. A review of adverse effects and benefits of nitrate and nitrite in drinking water and food on human health. Health Scope 2017, 6, 14164. [Google Scholar] [CrossRef] [Green Version]
- Manibalan, G.; Murugadoss, G.; Thangamuthu, R.; Ragupathy, P.; Kumar, M.R.; Kumar, R.M.; Jayavel, R. High electrochemical performance and enhanced electrocatalytic behavior of a hydrothermally synthesized highly crystalline heterostructure CeO2@NiO nanocomposite. Inorg. Chem. 2019, 58, 13843–13861. [Google Scholar]
- Keerthi, M.; Manavalan, S.; Chen S., M.; Shen P., W. A facile hydrothermal synthesis and electrochemical properties of manganese dioxide@graphitic carbon nitride nanocomposite toward highly sensitive detection of nitrite. J. Electrochem. Soc. 2019, 166, B1245–B1250. [Google Scholar] [CrossRef]
- Pasquali, C.E.; Hernando, L.P.F.A.; Alegria, J.S.D. Spectrophotometric simultaneous determination of nitrite, nitrate and ammonium in soils by flow injection analysis. Anal. Chim. Acta 2007, 600, 177–182. [Google Scholar]
- Ferrreira, I.; Silva, S. Quantification of residual nitrite and nitrate in ham by reverse-phase high performance liquid chromatography/diode array detector. Talanta 2008, 74, 1598–1602. [Google Scholar]
- Lin, Z.; Dou, X.G.; Li, H.F.; Ma, Y.; Lin, J.M. Nitrite sensing based on the carbon dots-enhanced chemiluminescence from peroxynitrous acid and carbonate. Talanta 2015, 132, 457–462. [Google Scholar] [CrossRef]
- Yang, H.; Xiang, Y.; Guo, X.; Wu, Y.; Wen, Y.; Yang, H. Diazo-reaction-based SERS substrates for detection of nitrite in saliva. Sens. Actuators B 2018, 271, 118–121. [Google Scholar]
- Jiang, C.; He, Y.; Liu, Y. Recent advances in sensors for electrochemical analysis of nitrate in food and environmental matrices. Analyst 2020, 145, 5400–5413. [Google Scholar] [CrossRef]
- Chang, T.E.; Chuang, C.H.; Kung, C.W. An iridium-deccrated metal-organic framework for electrocatalytic oxidation of nitrite. Electrochem. Commun. 2021, 122, 106899. [Google Scholar] [CrossRef]
- Shivakumar, M.; Manjunatha, S.; Nithyayini, K.N.; Dharmaprakash, M.S.; Nagashree, K.L. Electrocatalytic detection of nitrite at NiCo2O4 nanotapes synthesized via microwave-hydrothermal method. J. Electroanal. Chem. 2021, 882, 115016. [Google Scholar]
- Zhu, F.; Shi, H.; Wang, C.; Zhou, X.; Zhang, X.; Yang, F. Disposable carbon electrodes modified by a bismuth selenide/carboxylic multiwalled carbon nanotubes composite for the effective electrocatalytic analysis of nitrite. Sens. Actuators B 2021, 332, 129454. [Google Scholar] [CrossRef]
- Karimi-Takallo, A.; Dianat, S.; Hatefi-Mehrjardi, A. Fabrication and electrochemical study of K(1,1′-(1,4 Butanediyl) dipyridinium)2 [PW11O39Co(H2O)]/MWCNTs-COOH nanohybrid immobilized on glassy carbon for electrocatalytic detection of nitrite. J. Electroanal. Chem. 2021, 886, 115139. [Google Scholar] [CrossRef]
- Annalakshmi, M.; Kumaravel, S.; Chen, S.M.; Balasubramanian, P.; Balamurugan, T.S.T. A straightforward ultrasonic-assisted synthesis of zinc sulfide for supersensitive detection of carcinogenic nitrite ions in water samples. Sens. Actuators B 2020, 305, 127387. [Google Scholar] [CrossRef]
- Alam, M.S.; Shabik, M.F.; Rahman, M.M.; Valle, M.; Hasnat, M.A. Enhanced electrocatalytic effects of Pd particles immoilized on GC surface on the nitrite oxidation reactions. J. Electroanal. Chem. 2019, 839, 1–8. [Google Scholar] [CrossRef]
- Pila, T.; Chirawatkul, P.; Piyakeeratikul, P.; Somjit, V.; Sawangphruk, M.; Kongpatpanich, K. Metalloporohyrin-based metal-organic frameworks on flexible carbon paper for electrocatalytic nitrite oxidation. Chem. Eur. J. 2020, 26, 17399–17404. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Alagar, S.; Mariappan, R.; Piraman, S. Glassy carbon electrodes modified with reduced graphene oxide-MoS2-poly (3,4-ethylene dioxythiophene) nanocomposites for the non-enzymatic detection of nitrite in water and milk. Anal. Chim. Acta 2020, 1093, 93–105. [Google Scholar] [CrossRef]
- Asiri, A.M.; Adeosun, W.A.; Marwani, H.M.; Rahman, M.M. Homopolymerization of 3-aminobenzoic acid for enzyme-free electrocatalytic assay of nitrite ions. N. J. Chem. 2020, 44, 2022–2032. [Google Scholar] [CrossRef]
- Dong, S.; Li, Z.; Fu, Y.; Zhang, G.; Zhang, D.; Tong, M.; Huang, T. Bimetal-organic framework Cu-Ni-BTC and its derivative CuO@NiO: Construction of three environmental small-molecule electrochemical sensors. J. Electroanal. Chem. 2020, 858, 113785. [Google Scholar] [CrossRef]
- Choi, J.; Du, H.L.; Nguyen, C.K.; Suryanto, B.H.R.; Simonov, A.N.; MacFarlane, D.R. Electroreduction of nitrates, nitrites, and gaseous nitrogen oxides: A potential source of ammonia in dinitrogen reduction studies. ACS Energy. Lett. 2020, 5, 2095–2097. [Google Scholar] [CrossRef]
- Malko, D.; Kucernak, A.; Lopes, T. Performance of Fe-N/C oxygen reduction electrocatalysts toward NO2-, NO, and NH2OH electroreduction: From fundamental insights into the active center to a new method for environmental nitrite dustruction. J. Am. Chem. Soc. 2016, 138, 16056–16068. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, S.; Zhang, Z.; Tan, L.; Pang, H.; Ma, H. Facile fabrication of reduced graphene oxide and Keggin-type polyxometalates nanocomposite film for high performance electrocatalytic oxidation of nitrite. J. Electroanal. Chem. 2017, 807, 97–103. [Google Scholar] [CrossRef]
- Marlinda, A.R.; Pandikumar, A.; Yusoff, N.; Huang, N.M.; Lim, H.N. Electrochemical sensing of nitrite using a glassy carbon electrode modified with reduced functionalized graphene oxide decorated with flower-like zinc oxide. Microchim. Acta 2015, 182, 1113–1122. [Google Scholar] [CrossRef]
- Yang, B.; Bin, D.; Wang, H.; Zhu, M.; Yang, P.; Du, Y. High quality Pt-graphene nanocomposites for efficient electrocatalytic nitrite sensing. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 43–50. [Google Scholar] [CrossRef]
- Ge, Y.; Jamal, R.; Zhang, R.; Zhang, W.; Yu, Z.; Yan, Y.; Liu, Y.; Abdiryim, T. Electrochemical synthesis of multilayered PEDOT/PEDOT-SH/Au nanocomposites for electrochemial sensing of nitrite. Microchim. Acta 2020, 187, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Lin, H.; Zhang, X.; Qiu, F.; Yang, H. A glassy carbon electrode modified with carbon dots and gold nanoparticles for enhanced electrocatalytic oxidation and detection of nitrite. Microchim. Acta 2016, 183, 2807–2814. [Google Scholar] [CrossRef]
- Jian, J.M.; Fu, L.; Ji, J.; Lin, L.; Guo, X.; Ren, T. Electrochemically reduced graphene oxide/gold nanoparticles composite modified screen-printed carbon electrode for effective electrocatalytic analysis of nitrite in foods. Sens. Actuators B 2018, 262, 125–136. [Google Scholar] [CrossRef]
- Chen, T.; Xu, J.; Yang, P.; Sheng, Q.; Zheng, J.; Cao, W.; Yue, T.; Zhou, M.; Wang, C. Facile controlled synthesis of AuPd and Au Pt bimetallic nanocrystals for enhanced electrocatalytic sensing. Sens. Actuators B 2019, 298, 126724. [Google Scholar] [CrossRef]
- Hu, Y.J.; He, F.Y.; Ben, A.L.; Chen, C.Y. Synthesis of hollow Pt-Ni-graphene nanostructures for nonenzymatic glucose detection. J. Electroanal. Chem. 2014, 726, 55–61. [Google Scholar] [CrossRef]
- Yu, H.; Li, R.; Song, K.L. Amperometric determination of nitrite by using a nanocomposite prepared from gold nanoparticles, reduced graphene oxide and multi-walled carbon nanotubes. Microchim. Acta. 2019, 186, 624. [Google Scholar] [CrossRef]
- Wan, Y.; Zheng, Y.F.; Yin, H.Y.; Song, X.C. Au nanoparticle modified carbon paper electrode for an electrocatalytic oxidation nitrite sensor. N. J. Chem. 2016, 40, 3635–3641. [Google Scholar] [CrossRef]
- Shahamirifard, S.A.; Ghaedi, M. A new electrochemical sensor for simultaneous determination of arbutin and vitamin C based on hydroxyapatite- ZnO- Pd nanoparticles modified carbon paste electrode. Biosens. Bioelectro. 2019, 141, 111474. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Yuan, Y.; Leng, C.; Li, L.; Zhao, K.; Sun, Z. One-pot synthesis of noble metal/zinc oxide composites with controllable morphology and high catalytic performance. ACS Appl. Mater. Inter. 2017, 9, 16417–16425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Cai, X.L.; Song, L.Z.; Feng, F.Y.; Ding, J.Y.; Gong, F.L. 2D nanosheet-assembled Pd-ZnO microflowers for acetone sensor with enhanced performances. J. Phys. Chem. Solids 2019, 124, 330–335. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, H.J.; Koutavarapu, R.; Lee, S.H.; Arumugam, M.; Kim, J.H.; Choi, M.Y. ZnO supported Au/Pd bimetallic nanocomposites for plasmon improved photocatalytic activity for methylene blue degradation under visible light irradiation. Appl. Surf. Sci. 2019, 496, 143665. [Google Scholar] [CrossRef]
- Yue, X.; Luo, X.; Zhou, Z.; Wu, Y.; Bai, Y. pH-regulated synthesis of CuOx/ERGO nanohybrids with tunable electrocatalytic oxidation activity towards nitrite sensing. N. J. Chem. 2019, 43, 4947–4958. [Google Scholar] [CrossRef]
- Jain, G.; Montero, M.M.; Velusamy, T.; Maguire, P.; Mariotti, D. Porous zinc oxide nanocrystalline film deposition by atmospheric pressure plasma: Fabrication and energy band estimation. Plasma Process. Polym. 2017, 14, 1700052. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.; Yang, Z.; Navale, S.T.; Han, S.; Liu, X.; Liu, W.; Lu, Y.; Stadler, F.J.; Zhu, D. Ethanol sensing behavior of Pd-nanoparticles decorated ZnO-nanorod based chemresistive gas sensors. Sens. Actuators B 2019, 298, 126850. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, P.; Yin, Y.; Zhang, H.; Cai, C. Effects of structure, composition, and carbon support properties on the electrocatalytic activity of Pt-Ni-graphene nanocatalysts for the methanol oxidation. Appl. Catal. B Environ. 2012, 111–112, 208–217. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, P.K.; Sharma, A.; Satpati, A.K. Interfacial polymerized RGO/MnFe2O4/polyaniline fibrous nanocomposite supported glassy carbon electrode for selective and ultrasensitive detection of nitrite. Sens. Actuators B 2020, 309, 127763. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, J.; Du, X. Electrocatalytic oxidation of nitrite metal-free nitrogen-doped reduced graphene oxide nanosheets for sensitive detection. Talanta 2016, 155, 329–335. [Google Scholar] [CrossRef]






| Electrode | Linear Range (µM) | Detection Limit (μM) | Ref |
|---|---|---|---|
| rGO-MoS2-PEDOT | 1–1000 | 0.059 | [16] |
| Poly(3ABA) | 10–140 | 0.15 | [17] |
| PMo11V/PDDA-rGO | 0.125–1160 | 0.0028 | [21] |
| f-ZnO@rFGO | 100–3000 | 33 | [22] |
| Pt-RGO/GCE | 0.25–90 | 0.1 | [23] |
| CDs-Au-N | 0.1–2000 | 0.06 | [25] |
| ERGO/AuNPs | 1–6000 | 0.13 | [26] |
| AuPd | 2–4200 | 0.67 | [27] |
| AuNPs/CP | 1–100 | 0.093 | [30] |
| CuOx/ERGO | 0.1–100 | 0.072 | [35] |
| NrGO | 0.5–5000 | 0.2 | [40] |
| Pd-ZnO | 1–2350 | 0.2 | This work |
| Samples | Electrochemical Method (mg/kg) | Optical Method (mg/kg) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Sausage | 14.36 | 14.12 | 3.58 | 92.36 |
| 25.74 | 26.26 | 3.97 | 105.78 | |
| 15.68 | 15.97 | 4.13 | 93.46 | |
| Pickles | 8.79 | 8.23 | 4.56 | 108.56 |
| 12.33 | 11.98 | 3.98 | 105.38 | |
| 16.98 | 17.06 | 3.45 | 97.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; He, F.; Chen, C.; Zhang, C.; Liu, J. Facile Controlled Synthesis of Pd-ZnO Nanostructures for Nitrite Detection. Molecules 2023, 28, 99. https://doi.org/10.3390/molecules28010099
Hu Y, He F, Chen C, Zhang C, Liu J. Facile Controlled Synthesis of Pd-ZnO Nanostructures for Nitrite Detection. Molecules. 2023; 28(1):99. https://doi.org/10.3390/molecules28010099
Chicago/Turabian StyleHu, Yaojuan, Fengyun He, Changyun Chen, Changli Zhang, and Jingliang Liu. 2023. "Facile Controlled Synthesis of Pd-ZnO Nanostructures for Nitrite Detection" Molecules 28, no. 1: 99. https://doi.org/10.3390/molecules28010099
APA StyleHu, Y., He, F., Chen, C., Zhang, C., & Liu, J. (2023). Facile Controlled Synthesis of Pd-ZnO Nanostructures for Nitrite Detection. Molecules, 28(1), 99. https://doi.org/10.3390/molecules28010099