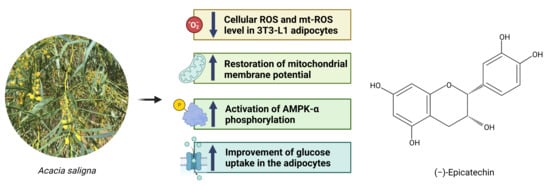
Antihyperglycemic Properties of Extracts and Isolated Compounds from Australian Acacia saligna on 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Profile of Extracts and Isolated Compounds
2.2. Viability Evaluation of the 3T3-L1 Adipocytes Treated with Isolated Compounds
2.3. Measurement of Cellular ROS Level
2.4. Measurement of Mt-ROS and MMP on Adipocytes
2.5. Cellular Glucose Uptake Assay
2.6. Immunoblot Analysis of AMPK Pathway Activation
3. Materials and Methods
3.1. Materials
3.2. Sample Collection, Extraction, Compound Isolation, and Molecular Elucidation
3.3. Cell Culture and Differentiation
3.4. Viability of 3T3-L1 Adipocytes
3.5. Determination of Cellular ROS Production
3.6. Cellular Glucose Uptake Assay
3.7. Mt-ROS Level Measurement
3.8. Mitochondrial Membrane Potential (MMP) Measurement
3.9. The Immunoblot Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Orchard, A.E.; Wilson, A.G. Flora of Australia: Mimosaceae, Acacia Part 2; CSIRO Melbourne: Clayton, Australia, 2001; Volume 11B, p. 475. [Google Scholar]
- Asmara, A.P.; Prasansuklab, A.; Tencomnao, T.; Ung, A.T. Identification of Phytochemicals in Bioactive Extracts of Acacia saligna Growing in Australia. Molecules 2023, 28, 1028. [Google Scholar] [CrossRef] [PubMed]
- Pessler, D.; Rudich, A.; Bashan, N. Oxidative stress impairs nuclear proteins binding to the insulin responsive element in the GLUT4 promoter. Diabetologia 2001, 44, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wang, C.C.; Huang, H.C.; Wei, Y.H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 2013, 280, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhitkovich, A. N-acetylcysteine: Antioxidant, aldehyde scavenger, and more. Chem. Res. Toxicol. 2019, 32, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, H.K.; Shim, W.; Anwar, M.A.; Kwon, J.W.; Kwon, H.K.; Kim, H.J.; Jeong, H.; Kim, H.M.; Hwang, D. Mechanism of cisplatin-induced cytotoxicity is correlated to impaired metabolism due to mitochondrial ROS generation. PLoS ONE 2015, 10, e0135083. [Google Scholar] [CrossRef]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic drugs and mitochondrial dysfunction: Focus on doxorubicin, trastuzumab, and sunitinib. Oxidative Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Woollard, A.C.S.; Wolff, S.P. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990, 268, 69–71. [Google Scholar] [CrossRef]
- Kuniyasu, A.; Tokunaga, M.; Yamamoto, T.; Inoue, S.; Obama, K.; Kawahara, K.; Nakayama, H. Oxidized LDL lysophosphatidylcholine stimulate plasminogen activator inhibitor-1 expression through reactive oxygen species generation and ERK1/2 activation in 3T3-L1 adipocytes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2011, 1811, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Al-Mana, F.; Al-Yafrsi, M.A. Antioxidant and biological activities of Acacia saligna and Lawsonia inermis natural populations. Plants 2020, 9, 908–925. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free. Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.F.; Rashid, S.; Rashid, S.M.; Ansari, M.A.; Khan, M.R.; Haq, N.; Alhareth, D.Y.; Ahmad, A.; Rehman, M.U. Naringenin regulates doxorubicin-induced liver dysfunction: Impact on oxidative stress and inflammation. Plants 2020, 9, 550–567. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, L.; Meng, B.; Zhou, X.; Wen, X.; Ren, D. Naringenin reduces oxidative stress and improves mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons. Int. J. Mol. Med. 2017, 40, 1582–1590. [Google Scholar] [CrossRef]
- Limasset, B.; Le Doucen, C.; Dore, J.C.; Ojasoo, T.; Damon, M.; De Paulet, A.C. Effects of flavonoids on the release of reactive oxygen species by stimulated human neutrophils: Multivariate analysis of structure-activity relationships (SAR). Biochem. Pharmacol. 1993, 46, 1257–1271. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Vicentini, F.T.M.C.; Baracat, M.M.; Georgetti, S.R.; Cardoso, R.D.R.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Fonseca, M.J.V.; Casagrande, R. Flavonoids as anti-inflammatory and analgesic drugs: Mechanisms of action and perspectives in the development of pharmaceutical forms. Stud. Nat. Prod. Chem. 2012, 36, 297–330. [Google Scholar]
- Lebiedzinska, M.; Karkucinska-Wieckowska, A.; Giorgi, C.; Karczmarewicz, E.; Pronicka, E.; Pinton, P.; Duszynski, J.; Pronicki, M.; Wieckowski, M.R. Oxidative stress-dependent p66Shc phosphorylation in skin fibroblasts of children with mitochondrial disorders. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 952–960. [Google Scholar] [CrossRef]
- Elefantova, K.; Lakatos, B.; Kubickova, J.; Sulova, Z.; Breier, A. Detection of the mitochondrial membrane potential by the cationic dye JC-1 in L1210 cells with massive overexpression of the plasma membrane ABCB1 drug transporter. Int. J. Mol. Sci. 2018, 19, 1985–1999. [Google Scholar] [CrossRef]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of mitochondrial reverse electron transport in ROS signaling: Potential roles in health and disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Batandier, C.; Guigas, B.; Detaille, D.; El-Mir, M.Y.; Fontaine, E.; Rigoulet, M.; Leverve, X.M. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J. Bioenerg. Biomembr. 2006, 38, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Vial, G.; Detaille, D.; Guigas, B. Role of mitochondria in the mechanism (s) of action of metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Akter, M.; Rahman, M.M.; Sikder, M.T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Investigating the protective actions of D-pinitol against arsenic-induced toxicity in PC12 cells and the underlying mechanism. Environ. Toxicol. Pharmacol. 2020, 74, 103302–103312. [Google Scholar] [CrossRef] [PubMed]
- López-Domènech, S.; Bañuls, C.; de Marañón, A.M.; Abab-Jiménez, Z.; Morillas, C.; Gómez-Abril, S.Á.; Rovira-Llopis, S.; Víctor, V.M.; Hernández-Mijares, A.; Rocha, M. Pinitol alleviates systemic inflammatory cytokines in human obesity by a mechanism involving unfolded protein response and sirtuin 1. Clin. Nutr. 2018, 37, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lim, Y.; Kwon, S.W.; Kwon, O. Pinitol consumption improves liver health status by reducing oxidative stress and fatty acid accumulation in subjects with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Nutr. Biochem. 2019, 68, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, T.; Muralidharan, A.R.; Joseph, T.; Hsu, M.J.; Thomas, P.A.; Geraldine, P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin–nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012, 68, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Sadler, D.G.; Barlow, J.; Draijer, R.; Jones, H.; Thijssen, D.H.J.; Stewart, C.E. (–)-Epicatechin alters reactive oxygen and nitrogen species production independent of mitochondrial respiration in human vascular endothelial cells. Oxidative Med. Cell. Longev. 2022, 2022, 4413191. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Cao, Z.; Li, W.; Liu, R.; Chen, Y.; Li, C.; Song, Y.; Liu, G.; Hu, J. Naringenin ameliorates homocysteine induced endothelial damage via the AMPKα/Sirt1 pathway. J. Adv. Res. 2021, 34, 137–147. [Google Scholar] [CrossRef]
- Gao, C.L.; Zhu, C.; Zhao, Y.P.; Chen, X.H.; Ji, C.B.; Zhang, C.M.; Zhu, J.G.; Xia, Z.K.; Tong, M.L.; Guo, X.R. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2010, 320, 25–33. [Google Scholar] [CrossRef]
- Silva Junior, J.A.; Silva, A.C.V.F.D.A.; Figueiredo, L.S.; Araujo, T.R.; Freitas, I.N.; Carneiro, E.M.; Ribeiro, E.S.; Ribeiro, R.A. D-Pinitol increases insulin secretion and regulates hepatic lipid metabolism in Msg-obese mice. An. Acad. Bras. Ciências 2020, 92, 1–14. [Google Scholar] [CrossRef]
- Pessler-Cohen, D.; Pekala, P.H.; Kovsan, J.; Bloch-Damti, A.; Rudich, A.; Bashan, N. GLUT4 repression in response to oxidative stress is associated with reciprocal alterations in C/EBP alpha and delta isoforms in 3T3-L1 adipocytes. Arch. Physiol. Biochem. 2006, 112, 3–12. [Google Scholar] [CrossRef]
- Hadrich, F.; Garcia, M.; Maalej, A.; Moldes, M.; Isoda, H.; Feve, B.; Sayadi, S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016, 151, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Nooron, N.; Athipornchai, A.; Suksamrarn, A.; Chiabchalard, A. Mahanine enhances the glucose-lowering mechanisms in skeletal muscle and adipocyte cells. Biochem. Biophys. Res. Commun. 2017, 494, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, M.E.; Kauffman, M.K.; Traore, K.; Zhu, H.; Trush, M.A.; Jia, Z.; Li, Y.R. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React. Oxyg. Species 2016, 2, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Harshkova, D.; Zielińska, E.; Aksmann, A. Optimization of a microplate reader method for the analysis of changes in mitochondrial membrane potential in Chlamydomonas reinhardtii cells using the fluorochrome JC-1. J. Appl. Phycol. 2019, 31, 3691–3697. [Google Scholar] [CrossRef]
- Lee, J.O.; Lee, S.K.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in 3T3-L1 preadipocyte cells. J. Biol. Chem. 2012, 287, 44121–44129. [Google Scholar] [CrossRef]






| Compounds | Extract/Amount (%w/w Extract) | Compound | Extract/Amount (%w/w Extract) |
|---|---|---|---|
 | FL-MeOH/1.75 |  | LF-MeOH/8 |
 | FL-MeOH/2.58 |  | LF-MeOH and BK-MeOH/0.9 b, 2.53 c |
 | FL/MeOH/1.52 |  | LF-MeOH/1 |
 | FL-MeOH and LF-MeOH/4.13 a, 2.68 b |  | LF-MeOH/5 |
 | FL-MeOH and BK-MeOH/2.5 a, 17.83 c |  | LF-MeOH/5 |
| Compound | % Viability of 3T3-L1 Adipocytes at Concentrations of (µM) | |||
|---|---|---|---|---|
| 15.63 | 31.25 | 62.5 | 125 | |
| Naringenin 1 | 93 ± 6 | 85 ± 6 | 87 ± 8 | 78 ± 2 ** |
| Compound 2 | 78 ± 4 * | 79 ± 6 * | 90 ± 6 | 90 ± 5 |
| Isosalipurposide 3 | 100 ± 4 | 101 ± 4 | 101 ± 4 | 101 ± 3 |
| Quercitrin 4 | 99 ± 1 | 99 ± 2 | 99 ± 2 | 100 ± 2 |
| D-(+)-pinitol 5a | 98 ± 5 | 99 ± 4 | 98 ± 3 | 98 ± 3 |
| (–)-Pinitol 5b | 94 ± 3 | 94 ± 4 | 94 ± 4 | 96 ± 4 |
| (–)-Epicatechin 6 | 89 ± 7 | 95 ± 2 | 92 ± 1 | 87 ± 7 * |
| 2,4-Di-tert-butylphenol 7 | 99 ± 1 | 82 ± 5 * | 89 ± 11 | 90 ± 8 |
| Myricitrin 8 | 105 ± 5 | 105 ± 5 | 103 ± 3 | 104 ± 4 |
| Compound 9 | 83 ± 3 * | 96 ± 3 | 90 ± 6 | 85 ± 5 * |
| Vehicle | 100 ± 1 | |||
| Sample | Mt-ROS Level (%) | J-Aggregates (Red)/JC-1 Monomers (Green) Percentage (%) | ||
|---|---|---|---|---|
| 12.5 μg/mL | 50 μg/mL | 12.5 μg/mL | 50 μg/mL | |
| Vehicle control | 100 ± 9 | 100 ± 10 | ||
| FL-MeOH | 103 ± 28 | 68 ± 27 | 138 ± 24 | 304 ± 8 *** |
| LF-MeOH | 98 ± 9 | 47 ± 20 | 97 ± 2 | 179 ± 19 |
| BK-MeOH | 103 ± 24 | 42 ± 4 | 124 ± 33 | 247 ± 29 * |
| Metformin 10 µM | 67 ± 2 | 207 ± 42 | ||
| Undifferentiated cells | 71 ± 8 | 191 ± 11 | ||
| Sample | Mt-ROS Level (%) | J Aggregates/JC-1 Monomers Percentage (%) | ||||
|---|---|---|---|---|---|---|
| 0.1 μM | 5 μM | 10 μM | 0.1 μM | 5 μM | 10 μM | |
| Vehicle control | 100 ± 9 | 100 ± 10 | ||||
| Naringenin 1 | 65 ± 6 | 65 ± 8 | 49 ± 11 ** | 95 ± 3 | 173 ± 13 | 267 ± 31 **** |
| Compound 2 | 72 ± 3 | 69 ± 3 | 57 ± 6 * | 107 ± 10 | 124 ± 17 | 206 ± 11 * |
| Isosalipurposide 3 | 78 ± 2 | 64 ± 5 | 57 ± 7 * | 89 ± 7 | 127 ± 8 | 164 ± 6 |
| Quercitrin 4 | 91± 2 | 72 ± 9 | 57 ± 4 * | 74 ± 12 | 105 ± 21 | 128 ± 7 |
| D-(+)-pinitol 5a | 71 ± 7 | 72 ± 3 | 56 ± 8 * | 121 ± 20 | 142 ± 16 | 301 ± 42 **** |
| (−)-Pinitol 5b | 103 ± 4 | 64 ± 5 | 55 ± 3 * | 196 ± 28 | 224 ± 29* | 238 ± 21 ** |
| (−)-Epicatechin 6 | 67 ± 7 | 56 ± 10 * | 46 ± 4 ** | 119± 13 | 120 ± 18 | 225 ± 32 ** |
| 2,4-Di-tert-buylphenol 7 | 73 ± 1 | 68 ± 7 | 46 ± 8 ** | 92 ± 5 | 148 ± 24 | 165 ± 9 |
| Myricitrin 8 | 84 ± 15 | 70 ± 17 | 68 ± 5 | 127 ± 14 | 132± 22 | 194 ± 14 |
| Compound 9 | 95 ± 18 | 89± 7 | 78 ± 8 | 148 ± 25 | 135 ± 15 | 187 ± 23 |
| Metformin 10 µM | 66 ± 2 | 207 ± 42 | ||||
| Undifferentiated cells | 71 ± 8 | 191 ± 11 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asmara, A.P.; Prasansuklab, A.; Chiabchalard, A.; Chen, H.; Ung, A.T. Antihyperglycemic Properties of Extracts and Isolated Compounds from Australian Acacia saligna on 3T3-L1 Adipocytes. Molecules 2023, 28, 4054. https://doi.org/10.3390/molecules28104054
Asmara AP, Prasansuklab A, Chiabchalard A, Chen H, Ung AT. Antihyperglycemic Properties of Extracts and Isolated Compounds from Australian Acacia saligna on 3T3-L1 Adipocytes. Molecules. 2023; 28(10):4054. https://doi.org/10.3390/molecules28104054
Chicago/Turabian StyleAsmara, Anjar P., Anchalee Prasansuklab, Anchalee Chiabchalard, Hui Chen, and Alison T. Ung. 2023. "Antihyperglycemic Properties of Extracts and Isolated Compounds from Australian Acacia saligna on 3T3-L1 Adipocytes" Molecules 28, no. 10: 4054. https://doi.org/10.3390/molecules28104054
APA StyleAsmara, A. P., Prasansuklab, A., Chiabchalard, A., Chen, H., & Ung, A. T. (2023). Antihyperglycemic Properties of Extracts and Isolated Compounds from Australian Acacia saligna on 3T3-L1 Adipocytes. Molecules, 28(10), 4054. https://doi.org/10.3390/molecules28104054