Transition Metal Sensing with Nitrogenated Holey Graphene: A First-Principles Investigation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Geometric Optimization
2.2. Most Stable Spin State of TM@C2N Complexes
2.3. Interaction Energies
2.4. Natural Bond Orbital Analysis (NBO)
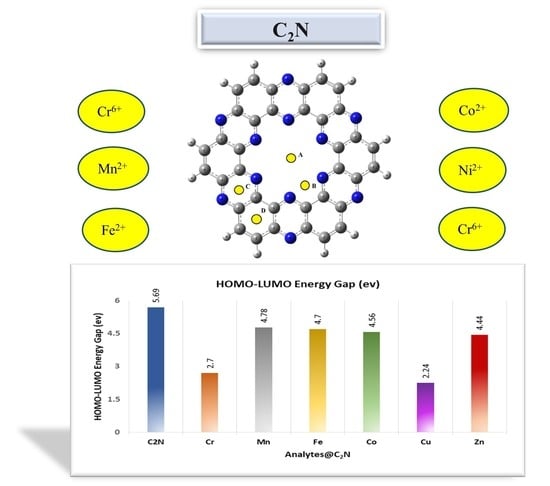
2.5. Frontier Molecular Orbital Analysis (FMO)
2.6. Non-Covalent Interactions (NCI)
2.7. QTAIM Analysis
2.8. SAPT0 Analysis
2.9. Electrical Conductivity and Sensitivity Analysis
3. Computational Methodology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pareek, B.; Singh, V.; Sharma, V.; Singh, P.S. Properties of Transition Metals and Their Compounds. Contemp. Adv. Sci. Technol. 2021, 1, 146. [Google Scholar]
- Hossain, M.R.; Hasan, M.M.; Nishat, M.; Noor, E.A.; Ahmed, F.; Ferdous, T.; Hossain, M.A. DFT and QTAIM investigations of the adsorption of chlormethine anticancer drug on the exterior surface of pristine and transition metal functionalized boron nitride fullerene. J. Mol. Liq. 2021, 323, 114627. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; El-Bindary, A.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, M.A. Synthesis, characterization, design, molecular docking, anti COVID-19 activity, DFT calculations of novel Schiff base with some transition metal complexes. J. Mol. Liq. 2022, 346, 117850. [Google Scholar] [CrossRef]
- Bönnemann, H.; Brijoux, W.; Brinkmann, R.; Joußen, T.; Korall, B.; Dinjus, E. Formation of colloidal transition metals in organic phases and their application in catalysis. Angew. Chem. Int. Ed. Engl. 1991, 30, 1312–1314. [Google Scholar] [CrossRef]
- Ullah, F.; Ayub, K.; Mahmood, T. High performance SACs for HER process using late first-row transition metals anchored on graphyne support: A DFT insight. Int. J. Hydrogen Energy 2021, 46, 37814–37823. [Google Scholar] [CrossRef]
- O’Halloran, T.V. Transition metals in control of gene expression. Science 1993, 261, 715–725. [Google Scholar] [CrossRef]
- Huang, Y.W.; Wu, C.H.; Aronstam, R.S. Toxicity of Transition Metal Oxide Nanoparticles: Recent Insights from in vitro Studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef]
- Crans, D.C.; Kostenkova, K. Open questions on the biological roles of first-row transition metals. Commun. Chem. 2020, 3, 104. [Google Scholar] [CrossRef]
- Mills, K.C.; Curry, S.C. Acute iron poisoning. Emerg. Med. Clin. N. Am. 1994, 12, 397–413. [Google Scholar] [CrossRef]
- Van Cleave, C.; Crans, D.C. The first-row transition metals in the periodic table of medicine. Inorganics 2019, 7, 111. [Google Scholar] [CrossRef]
- Reaney, S.H.; Kwik-Uribe, C.L.; Smith, D.R. Manganese oxidation state and its implications for toxicity. Chem. Res. Toxicol. 2002, 15, 1119–1126. [Google Scholar] [CrossRef]
- Erikson, K.M.; Syversen, T.; Aschner, J.L.; Aschner, M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ. Toxicol. Pharmacol. 2005, 19, 415–421. [Google Scholar] [CrossRef]
- Crossgrove, J.; Zheng, W. Manganese toxicity upon overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef]
- McMillan, D. A brief history of the neurobehavioral toxicity of manganese: Some unanswered questions. Neurotoxicology 1999, 20, 499–507. [Google Scholar]
- Dias, A.M.; Hussain, A.; Marcos, A.S.; Roque, A.C. A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnol. Adv. 2011, 29, 142–155. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I. Iron toxicity and antioxidant nutrients. Toxicology 2002, 180, 23–32. [Google Scholar] [CrossRef]
- Chamnongpol, S.; Dodson, W.; Cromie, M.J.; Harris, Z.L.; Groisman, E.A. Fe(III)-mediated cellular toxicity. Mol. Microbiol. 2002, 45, 711–719. [Google Scholar] [CrossRef]
- Britton, R.S.; Leicester, K.L.; Bacon, B.R. Iron toxicity and chelation therapy. Int. J. Hematol. 2002, 76, 219–228. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Suh, M.; Thompson, C.M.; Brorby, G.P.; Mittal, L.; Proctor, D.M. Inhalation cancer risk assessment of cobalt metal. Regul. Toxicol. Pharm. 2016, 79, 74–82. [Google Scholar] [CrossRef]
- Kaim, W.; Rall, J. Copper—A “modern” bioelement. Angew. Chem. Int. Ed. Engl. 1996, 35, 43–60. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Frassinetti, S.; Bronzetti, G.; Caltavuturo, L.; Cini, M.; Croce, C.D. The role of zinc in life: A review. J. Env. Pathol. Toxicol. Oncol. 2006, 25, 597–610. [Google Scholar] [CrossRef]
- Beyersmann, D. Homeostasis and cellular functions of zinc. Materialwiss. Werkstofftech. 2002, 33, 764–769. [Google Scholar] [CrossRef]
- Tudor, R.; Zalewski, P.D.; Ratnaike, R.N. Zinc in health and chronic disease. J. Nutr. Health Aging 2005, 9, 45–51. [Google Scholar]
- Shekhawat, K.; Chatterjee, S.; Joshi, B. Chromium toxicity and its health hazards. Int. J. Adv. Res 2015, 3, 167–172. [Google Scholar]
- Guertin, J. Toxicity and health effects of chromium (all oxidation states). In Chromium (VI) Handbook; CRC Press: Boca Raton, FL, USA, 2004; pp. 215–234. [Google Scholar] [CrossRef]
- Gu, X.; Evans, L.J. Surface complexation modelling of Cd (II), Cu (II), Ni (II), Pb (II) and Zn (II) adsorption onto kaolinite. Geochim. Cosmochim. Acta 2008, 72, 267–276. [Google Scholar] [CrossRef]
- Mulani, K.; Daniels, S.; Rajdeo, K.; Tambe, S.; Chavan, N. Adsorption of chromium (VI) from aqueous solutions by coffee polyphenol-formaldehyde/acetaldehyde resins. J. Polym. 2013, 2013, 98368. [Google Scholar] [CrossRef]
- Saha, B.; Tai, M.; Streat, M. Adsorption of transition metals from aqueous solutions by modified activated carbons. Chem. Eng. Res. Des. 2003, 81, 1343–1353. [Google Scholar] [CrossRef]
- White, R.L.; White, C.M.; Turgut, H.; Massoud, A.; Tian, Z.R. Comparative studies on copper adsorption by graphene oxide and functionalized graphene oxide nanoparticles. J. Taiwan Inst. Chem. Eng. 2018, 85, 18–28. [Google Scholar] [CrossRef]
- Yang, L.; Huang, N.; Huang, L.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. An electrochemical sensor for highly sensitive detection of copper ions based on a new molecular probe Pi-A decorated on graphene. Anal. Methods 2017, 9, 618–624. [Google Scholar] [CrossRef]
- Das, B.; Ghosh, A.; Dorairaj, D.P.; Dolai, M.; Karvembu, R.; Mabhai, S.; Im, H.; Dey, S.; Jana, A.; Misra, A. Multiple ion (Al3+, Cr3+, Fe3+, and Cu2+) sensing using a cell-compatible rhodamine-phenolphthalein-derived Schiff-base probe. J. Mol. Liq. 2022, 354, 118824. [Google Scholar] [CrossRef]
- Yar, M.; Hashmi, M.A.; Khan, A.; Ayub, K. Carbon nitride 2-D surface as a highly selective electrochemical sensor for V-series nerve agents. J. Mol. Liq. 2020, 311, 113357. [Google Scholar] [CrossRef]
- Yar, M.; Hashmi, M.A.; Ayub, K. The C 2 N surface as a highly selective sensor for the detection of nitrogen iodide from a mixture of NX 3 (X = Cl, Br, I) explosives. RSC Adv. 2020, 10, 31997–32010. [Google Scholar] [CrossRef]
- Csonka, G. Proper basis set for quantum mechanical studies of potential energy surfaces of carbohydrates. J. Mol. Struct. Theochem. 2002, 584, 1–4. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of Density Functionals by Combining the Method of Constraint Satisfaction with Parametrization for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Burns, L.A.; Vazquez-Mayagoitia, A.; Sumpter, B.G.; Sherrill, C.D. Density-functional approaches to noncovalent interactions: A comparison of dispersion corrections (DFT-D), exchange-hole dipole moment (XDM) theory, and specialized functionals. J. Chem. Phys. 2011, 134, 084107. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the Performance of the M05-2X and M06-2X Exchange-Correlation Functionals for Noncovalent Interactions in Biomolecules. J. Chem. Theory. Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef]
- Goel, S.; Masunov, A.E. Dissociation curves and binding energies of diatomic transition metal carbides from density functional theory. Int. J. Quantum Chem. 2011, 111, 4276–4287. [Google Scholar] [CrossRef]
- Malloum, A.; Conradie, J. Structures, binding energies and non-covalent interactions of furan clusters. J. Mol. Graph. Model. 2022, 111, 108102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Applications and validations of the Minnesota density functionals. Chem. Phys. Lett. 2011, 502, 1–13. [Google Scholar] [CrossRef]
- Elm, J.; Mikkelsen, K.V. Computational approaches for efficiently modelling of small atmospheric clusters. Chem. Phys. Lett. 2014, 615, 26–29. [Google Scholar] [CrossRef]
- Li, W.; Xie, H.; Huang, Y.; Song, L.; Shao, Y.; Qiu, K. Application of Gaussian 09/GaussView 5.0 in analytical chemistry teaching. J. Kunming Med. Univ. 2016, 134–136. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Vega, D.; Almeida, D. AIM-UC: An application for QTAIM analysis. J. Comput. Methods Sci. Eng. 2014, 14, 131–136. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Sherrill, C.D. Density fitting of intramonomer correlation effects in symmetry-adapted perturbation theory. J. Chem. Phys. 2010, 133, 014101. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Simmonett, A.C.; Parrish, R.M.; Schieber, M.C.; Galvelis, R.; Kraus, P.; Kruse, H.; Di Remigio, R.; Alenaizan, A.; et al. Psi4 1.4: Open-source software for high-throughput quantum chemistry. J. Chem. Phys. 2020, 152, 184108. [Google Scholar] [CrossRef]







| Spin States | Complexes | ||
|---|---|---|---|
| Cu2+@C2N | Co2+@C2N | Mn2+@C2N | |
| Doublet | 0.0 | 23 | 68 |
| Quartet | 37 | 0.0 | 69 |
| Sextet | 85 | 41 | 0.0 |
| Octet | 136 | 89 | 46 |
| Cr6+@C2N | Fe2+@C2N | Zn2+@C2N | |
| Singlet | 80 | 60 | 0.0 |
| Triplet | 0.0 | 21 | 28 |
| Quintet | 0.4 | 0.0 | 29 |
| Septet | 6.8 | 35 | 30 |
| TM@C2N (Solvent = Water) | Interaction Energy (kcal mol−1) | Interacting Atoms | Interaction Distance (Å) |
|---|---|---|---|
| Cu2+@C2N (Doublet) | −6.6 | Cu-61………N-47 | 2.21 |
| Cr6+@C2N (Quintet) | −9.2 | Cr-61………..N-34 | 2.09 |
| Zn2+@C2N (Singlet) | −15.9 | Zn-61………N-47 | 2.12 |
| Co2+@C2N (Quartet) | −20.7 | Co-61………N-34 | 2.20 |
| Fe2+@C2N (Sextet) | −25.9 | Fe-61………N-47 | 2.30 |
| Mn2+@C2N (Triplet) | −43.1 | Mn-61……..N-47 | 2.34 |
| Complexes | EHOMO | ELUMO | EH–L (eV) | QNBO |
|---|---|---|---|---|
| C2N | −7.870 | −2.17 | 5.71 | - |
| Cu2+@C2N | −13.30 | −11.06 | 2.24 | 0.871 |
| Fe2+@C2N | −13.22 | −8.51 | 4.71 | 1.640 |
| Mn2+@C2N | −13.27 | −8.48 | 4.80 | 1.672 |
| Zn2+@C2N | −13.17 | −8.73 | 4.44 | 1.652 |
| Cr6+@C2N | −24.05 | −21.35 | 2.70 | 1.79 |
| Co2+@C2N | −13.20 | −8.637 | 4.56 | 0.861 |
| Analytes@C2N | C2N—Analyte | BCP | ρ | ∇ 2ρ | G (r) | V (r) | H (r) | V(r)/G(r) |
|---|---|---|---|---|---|---|---|---|
| Cu2+@C2N | N18- | 96 | 0.02 | 0.07 | 0.01 | −0.01 | 0.001 | 1.0 |
| N23- | 99 | 0.05 | 0.19 | 0.06 | −0.08 | −0.014 | 1.3 | |
| N36- | 112 | 0.05 | 0.19 | 0.06 | −0.07 | −0.014 | 1.1 | |
| N44- | 117 | 0.02 | 0.07 | 0.01 | −0.04 | 0.001 | 4 | |
| Fe2+@C2N | N18- | 97 | 0.35 | −0.10 | 0.34 | −0.94 | −0.59 | 2.7 |
| N23- | 100 | 0.33 | −0.97 | 0.11 | −0.48 | −0.36 | 4.3 | |
| N25- | 104 | 0.29 | −0.78 | 0.07 | −0.34 | −0.27 | 4.8 | |
| N37- | 115 | 0.30 | −0.82 | 0.08 | −0.37 | −0.29 | 4.6 | |
| N39- | 119 | 0.34 | −0.10 | 0.31 | −0.89 | −0.57 | 2.8 | |
| N-44 | 122 | 0.02 | 0.18 | 0.04 | −0.03 | 0.008 | 0.7 | |
| Zn2+@C2N | N20- | 97 | 0.40 | −0.010 | 0.34 | −0.90 | −0.6 | 2.6 |
| N21- | 101 | 0.29 | −0.779 | 0.078 | −0.35 | −0.3 | 4.4 | |
| N33- | 109 | 0.29 | −0.114 | 0.033 | 0.35 | −0.3 | 10 | |
| N42- | 115 | 0.302 | −0.82 | 0.086 | −0.37 | −0.2 | 4.3 | |
| Mn2+@C2N | N19- | 97 | 0.35 | −0.10 | 0.34 | −0.94 | −0.6 | 2.7 |
| N22- | 101 | 0.293 | −0.779 | 0.078 | −0.35 | −0.3 | 4.4 | |
| N28- | 106 | 0.292 | −0.11 | 0.032 | −0.35 | −0.3 | 11 | |
| N34- | 111 | 0.28 | −0.76 | 0.073 | −0.33 | −0.3 | 4.5 | |
| N40- | 116 | 0.33 | −0.99 | 0.122 | −0.49 | −0.4 | 4.0 | |
| N43- | 120 | 0.015 | 0.045 | 0.010 | −0.009 | 0.008 | 0.9 | |
| Cr6+@C2N | N18- | 94 | 0.016 | 0.033 | 0.010 | −0.012 | −0.002 | 1.2 |
| N23- | 97 | 0.053 | 0.29 | 0.071 | −0.069 | 0.003 | 0.9 | |
| N36- | 108 | 0.069 | 0.375 | 0.095 | −0.097 | −0.002 | 1.0 | |
| N44- | 112 | 0.026 | 0.068 | 0.020 | −0.024 | 0.004 | 1.2 | |
| Co2+@C2N | N18- | 98 | 0.02 | 0.05 | 0.016 | −0.019 | −0.003 | 1.1 |
| N26- | 103 | 0.057 | 0.18 | 0.059 | −0.072 | −0.013 | 1.2 | |
| N39- | 116 | 0.057 | 0.18 | 0.059 | −0.072 | −0.013 | 1.2 | |
| N44- | 117 | 0.021 | 0.0561 | 0.016 | −0.019 | −0.002 | 1.1 |
| TM@C2N | Eelec | Eexch | EInd | Edisp | ESAPT0 |
|---|---|---|---|---|---|
| Cu2+@C2N | −167.46 | 41.33 | −121.82 | −7.15 | −255.09 |
| Fe2+@C2N | −163.28 | 38.03 | 0.3244 | −6.96 | −131.88 |
| Mn2+@C2N | −156.84 | 24.55 | −144.44 | −6.62 | −283.35 |
| Zn2+@C2N | −173.33 | 45.06 | −170.61 | −7.31 | −306.20 |
| Co2+@C2N | −172.68 | 54.46 | −48.38 | −7.82 | −174.43 |
| Cr6+@C2N | −463.26 | 81.73 | −4759.46 | −4.76 | −5145.76 |
| Complexes | Φ | S (%) | σ |
|---|---|---|---|
| C2N | 5.02 | - | 1.09 × 10−48 |
| Cu2+@C2N | 12.18 | 142.63 | 1.53 × 10−19 |
| Fe2+@C2N | 10.86 | 10.79 | 2.74 × 10−40 |
| Mn2+@C2N | 10.87 | 0.09 | 4.80 × 10−41 |
| Zn2+@C2N | 10.95 | 0.69 | 5.08 × 10−38 |
| Cr6+@C2N | 22.7 | 107.30 | 2.09 × 10−23 |
| Co2+@C2N | 10.92 | 51.90 | 4.98 × 10−39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, U.; Ullah, F.; Binti Zainal Arfan, N.H.; Abdul Hamid, M.H.S.; Mahmood, T.; Sheikh, N.S.; Ayub, K. Transition Metal Sensing with Nitrogenated Holey Graphene: A First-Principles Investigation. Molecules 2023, 28, 4060. https://doi.org/10.3390/molecules28104060
Sohail U, Ullah F, Binti Zainal Arfan NH, Abdul Hamid MHS, Mahmood T, Sheikh NS, Ayub K. Transition Metal Sensing with Nitrogenated Holey Graphene: A First-Principles Investigation. Molecules. 2023; 28(10):4060. https://doi.org/10.3390/molecules28104060
Chicago/Turabian StyleSohail, Uroosa, Faizan Ullah, Nur Hazimah Binti Zainal Arfan, Malai Haniti Sheikh Abdul Hamid, Tariq Mahmood, Nadeem S. Sheikh, and Khurshid Ayub. 2023. "Transition Metal Sensing with Nitrogenated Holey Graphene: A First-Principles Investigation" Molecules 28, no. 10: 4060. https://doi.org/10.3390/molecules28104060
APA StyleSohail, U., Ullah, F., Binti Zainal Arfan, N. H., Abdul Hamid, M. H. S., Mahmood, T., Sheikh, N. S., & Ayub, K. (2023). Transition Metal Sensing with Nitrogenated Holey Graphene: A First-Principles Investigation. Molecules, 28(10), 4060. https://doi.org/10.3390/molecules28104060