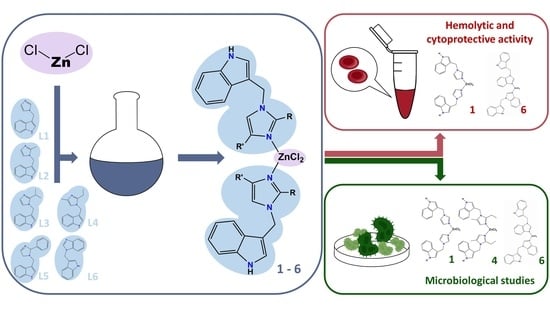
Synthesis, Structure and Biological Activity of Indole–Imidazole Complexes with ZnCl2: Can Coordination Enhance the Functionality of Bioactive Ligands?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterization of ZnCl2 Complexes with Indole–Imidazole Ligands
2.2. X-ray Analysis
2.3. Biological Activity
2.3.1. Hemolytic Activity
2.3.2. Cytoprotective Activity against Free Radicals
2.4. Antibacterial Study
2.5. Fungicidal Activity
2.6. In Silico Study
3. Materials and Methods
3.1. Instrumentation and Chemicals
3.2. Synthesis of Gramine Derivatives
3.3. X-ray Analysis
3.4. Biological Activity
3.4.1. Human Erythrocyte
3.4.2. Hemolytic Activity
3.4.3. Protective Activity against Oxidative Stress-Induced Hemolysis
3.4.4. Statistical Analysis
3.5. Antibacterial and Antifungal Activity Measurements
3.6. In Silico Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin Biosynthesis and Storage Forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Abdul-Hameed, Z.H.; Alotaibi, M.O.; Bawakid, N.O.; Sobahi, T.R.; Abdel-Lateff, A.; Alarif, W.M. Chemical Diversity and Bioactivities of Monoterpene Indole Alkaloids (MIAs) from Six Apocynaceae Genera. Molecules 2021, 26, 488. [Google Scholar] [CrossRef]
- Danilovich, M.E.; Alberto, M.R.; Juárez Tomás, M.S. Microbial Production of Beneficial Indoleamines (Serotonin and Melatonin) with Potential Application to Biotechnological Products for Human Health. J. Appl. Microbiol. 2021, 131, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Ditz, K.M.; Singletary, G.W.; Leland, T.J. Gramine Accumulation in Leaves of Barley Grown under High-Temperature Stress. Plant Physiol. 1983, 71, 896–904. [Google Scholar] [CrossRef]
- Kumar, S. A Brief Review of the Biological Potential of Indole Derivatives. Future J. Pharm. Sci. 2020, 6, 121. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Demurtas, M.; Baldisserotto, A.; Lampronti, I.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Indole Derivatives as Multifunctional Drugs: Synthesis and Evaluation of Antioxidant, Photoprotective and Antiproliferative Activity of Indole Hydrazones. Bioorg. Chem. 2019, 85, 568–576. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, Antioxidant and Cytoprotective Activity Evaluation of C-3 Substituted Indole Derivatives. Sci. Rep. 2021, 11, 15425. [Google Scholar] [CrossRef] [PubMed]
- Biradar, J.S.; Sasidhar, B.S.; Parveen, R. Synthesis, Antioxidant and DNA Cleavage Activities of Novel Indole Derivatives. Eur. J. Med. Chem. 2010, 45, 4074–4078. [Google Scholar] [CrossRef]
- Shirinzadeh, H.; Neuhaus, E.; Ince Erguc, E.; Tascioglu Aliyev, A.; Gurer-Orhan, H.; Suzen, S. New Indole-7-Aldehyde Derivatives as Melatonin Analogues; Synthesis and Screening Their Antioxidant and Anticancer Potential. Bioorg. Chem. 2020, 104, 104219. [Google Scholar] [CrossRef]
- Nieto, M.J.; Lupton, H.K. Indole and Indoline Scaffolds in Antimicrobials: Overview, Synthesis and Recent Advances in Antimicrobial Research. Curr. Med. Chem. 2021, 28, 4828–4844. [Google Scholar] [CrossRef]
- Tolomeu, H.V.; Fraga, C.A.M. Imidazole: Synthesis, Funcjonalization and Physicochemical Properties of a Privileged Structure in Medicinal Chemistry. Molecules 2023, 28, 838. [Google Scholar] [CrossRef] [PubMed]
- Siwach, A.; Verma, P.K. Synthesis and therapeutic potential of imidazole containing compounds. BMC Chem. 2021, 15, 12. [Google Scholar] [CrossRef]
- Verma, A.; Joshi, S.; Singh, D. Imidazole: Having Versatile Biological Activities. J. Chem. 2013, 2013, 329412. [Google Scholar] [CrossRef]
- Naureen, S.; Ijaz, F.; Munawar, M.A.; Asif, N.; Chaudhry, F.; Ashraf, M.; Khan, M.A.; Naureen, S.; Ijaz, F.; Munawar, M.A.; et al. Synthesis of Tetrasubstitutd Imidazoles Containing Indole and Their Antiurease and Antioxidant Activities. J. Chil. Chem. 2017, 62, 3583–3587. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Babijczuk, K.; Warżajtis, B.; Rychlewska, U.; Starzyk, J.; Cofta, G.; Mrówczyńska, L. Indole Derivatives Bearing Imidazole, Benzothiazole-2-Thione or Benzoxazole-2-Thione Moieties—Synthesis, Structure and Evaluation of Their Cytoprotective, Antioxidant, Antibacterial and Fungicidal Activities. Molecules 2023, 28, 708. [Google Scholar] [CrossRef]
- Bolous, M.; Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Maruoka, K.; Antharam, V.C.; Thangamani, S. Broad-Spectrum Antifungal Activity of Spirooxindolo-Pyrrolidine Tethered Indole/Imidazole Hybrid Heterocycles against Fungal Pathogens. Bioorg. Med. Chem. Lett. 2019, 29, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Benkli, K.; Demirayak, S.; Gundogdu-Karaburun, N.; Kiraz, N.; Iscan, G.; Ucucu, U. Synthesis and Antimicrobial Activities of Some Imidazole Substituted Indoles; NISCAIR-CSIR: New Delhi, India, 2004. [Google Scholar]
- Sumiya, T.; Ishigaki, M.; Oh, K. Synthesis of Imidazole and Indole Hybrid Molecules and Antifungal Activity against Rice Blast. Int. J. Chem. Eng. Appl. 2017, 8, 233–236. [Google Scholar] [CrossRef]
- James, D.A.; Koya, K.; Li, H.; Chen, S.; Xia, Z.; Ying, W.; Wu, Y.; Sun, L. Conjugated Indole-Imidazole Derivatives Displaying Cytotoxicity against Multidrug Resistant Cancer Cell Lines. Bioorg. Med. Chem. Lett. 2006, 16, 5164–5168. [Google Scholar] [CrossRef]
- Naureen, S.; Chaudhry, F.; Munawar, M.A.; Ashraf, M.; Hamid, S.; Khan, M.A. Biological Evaluation of New Imidazole Derivatives Tethered with Indole Moiety as Potent α-Glucosidase Inhibitors. Bioorg. Chem. 2018, 76, 365–369. [Google Scholar] [CrossRef]
- Hogendorf, A.S.; Hogendorf, A.; Popiołek-Barczyk, K.; Ciechanowska, A.; Mika, J.; Satała, G.; Walczak, M.; Latacz, G.; Handzlik, J.; Kieć-Kononowicz, K.; et al. Fluorinated Indole-Imidazole Conjugates: Selective Orally Bioavailable 5-HT7 Receptor Low-Basicity Agonists, Potential Neuropathic Painkillers. Eur. J. Med. Chem. 2019, 170, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, R.K.; Dash, M.; Mishra, U.K.; Mahapatra, A.; Dash, D.C. Synthesis, Spectral Characterization, and Fungicidal Activity of Transition Metal Complexes with Benzimidazolyl-2-Hydrazones of Glyoxal, Diacetyl, and Benzil. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2014, 44, 642–648. [Google Scholar] [CrossRef]
- Krishna, G.A.; Dhanya, T.M.; Shanty, A.A.; Raghu, K.G.; Mohanan, P.V. Transition Metal Complexes of Imidazole Derived Schiff Bases: Antioxidant/Anti-Inflammatory/Antimicrobial/Enzyme Inhibition and Cytotoxicity Properties. J. Mol. Struct. 2023, 1274, 134384. [Google Scholar] [CrossRef]
- Reshma, R.; Selwin Joseyphus, R.; Arish, D.; Reshmi Jaya, R.J.; Johnson, J. Tridentate Imidazole-Based Schiff Base Metal Complexes: Molecular Docking, Structural and Biological Studies. J. Biomol. Struct. Dyn. 2022, 40, 8602–8614. [Google Scholar] [CrossRef]
- Poyraz, M.; Sarı, M.; Güney, A.; Demirci, F.; Demirayak, S.; Şahin, E. Synthesis, Characterization and Antimicrobial Activity of a Zn(II) Complex with 1-(1H-Benzoimidazol-2-Yl)-Ethanone Thiosemicarbazone. J. Coord. Chem. 2008, 61, 3276–3283. [Google Scholar] [CrossRef]
- Li, C.-G.; Chai, Y.-M.; Chai, L.-Q.; Xu, L.-Y. Novel Zinc (II) and Nickel (II) Complexes of a Quinazoline-Based Ligand with an Imidazole Ring: Synthesis, Spectroscopic Property, Antibacterial Activities, Time-Dependent Density Functional Theory Calculations and Hirshfeld Surface Analysis. Appl. Organomet. Chem. 2022, 36, e6622. [Google Scholar] [CrossRef]
- Ismael, M.; Abdou, A.; Abdel-Mawgoud, A.-M. Synthesis, Characterization, Modeling, and Antimicrobial Activity of FeIII, CoII, NiII, CuII, and ZnII Complexes Based on Tri-Substituted Imidazole Ligand. Z. Anorg. Allg. Chem. 2018, 644, 1203–1214. [Google Scholar] [CrossRef]
- Pan, R.-K.; Song, J.-L.; Li, G.-B.; Lin, S.-Q.; Liu, S.-G.; Yang, G.-Z. Copper(II), Cobalt(II) and Zinc(II) Complexes Based on a Tridentate Bis(Benzimidazole)Pyridine Ligand: Synthesis, Crystal Structures, Electrochemical Properties and Antitumour Activities. Transit. Met. Chem. 2017, 42, 253–262. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S.; Zhao, D.; Chen, S.; Hu, J. Metal and Structure Tuned in Vitro Antitumor Activity of Benzimidazole-Based Copper and Zinc Complexes. J. Coord. Chem. 2013, 66, 1650–1660. [Google Scholar] [CrossRef]
- Shen, K.; Han, X.; Li, C.; Huang, G.; Mao, S.; Shi, X.; Wu, H. Synthesis, Structure, Electrochemical Properties, and Antioxidant Activities of Copper(II) and Zinc(II) Complexes with N,N-Bis(N-Ethyl-2-Ylmethylbenzimidazol)Allylamine Ligand. J. Coord. Chem. 2018, 71, 980–990. [Google Scholar] [CrossRef]
- Wu, H.; Yuan, J.; Zhang, Y.; Shi, F.; Pan, G.; Kong, J.; Fan, X. Synthesis, Crystal Structure, DNA-Binding Properties and Antioxidant Activity of Zinc(II) Complexes Based on the V-Shaped Bis(2-Benzimidazol-2-Ylmethyl)Benzylamine Ligand and Its Derivative. Inorg. Chim. Acta 2013, 404, 13–22. [Google Scholar] [CrossRef]
- Benhassine, A.; Boulebd, H.; Anak, B.; Bouraiou, A.; Bouacida, S.; Bencharif, M.; Belfaitah, A. Copper(II) and Zinc(II) as Metal-Carboxylate Coordination Complexes Based on (1-Methyl-1H-Benzo[d]Imidazol-2-Yl) Methanol Derivative: Synthesis, Crystal Structure, Spectroscopy, DFT Calculations and Antioxidant Activity. J. Mol. Struct. 2018, 1160, 406–414. [Google Scholar] [CrossRef]
- Ling, N.; Wang, X.; Zeng, D.; Zhang, Y.-W.; Fang, X.; Yang, H.-X. Synthesis, Characterization and Biological Assay of Three New Benzotriazole-Based Zn(II) Complexes. J. Mol. Struct. 2020, 1206, 127641. [Google Scholar] [CrossRef]
- Yernale, N.G.; Matada, B.S.; Vibhutimath, G.B.; Biradar, V.D.; Karekal, M.R.; Udayagiri, M.D.; Hire Mathada, M.B. Indole Core-Based Copper(II), Cobalt(II), Nickel(II) and Zinc(II) Complexes: Synthesis, Spectral and Biological Study. J. Mol. Struct. 2022, 1248, 131410. [Google Scholar] [CrossRef]
- Haribabu, J.; Jeyalakshmi, K.; Arun, Y.; Bhuvanesh, N.S.P.; Perumal, P.T.; Karvembu, R. Synthesis of Ni(II) Complexes Bearing Indole-Based Thiosemicarbazone Ligands for Interaction with Biomolecules and Some Biological Applications. J. Biol. Inorg. Chem. 2017, 22, 461–480. [Google Scholar] [CrossRef]
- Dillon, C.T.; Hambley, T.W.; Kennedy, B.J.; Lay, P.A.; Zhou, Q.; Davies, N.M.; Biffin, J.R.; Regtop, H.L. Gastrointestinal Toxicity, Antiinflammatory Activity, and Superoxide Dismutase Activity of Copper and Zinc Complexes of the Antiinflammatory Drug Indomethacin. Chem. Res. Toxicol. 2003, 16, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Mediratta, D.K.; Pathak, K. Bioavailability of Indomethacin from Zinc-Indomethacin Complex. Int. J. Pharm. 1990, 60, 27–33. [Google Scholar] [CrossRef]
- Singla, A.K.; Wadhwa, H. Zinc-Indomethacin Complex: Synthesis, Physicochemical and Biological Evaluation in the Rat. Int. J. Pharm. 1995, 120, 145–155. [Google Scholar] [CrossRef]
- McCall, K.A.; Huang, C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef]
- Loomans, H.J.; Hahn, B.L.; Li, Q.Q.; Phadnis, S.H.; Sohnle, P.G. Histidine-Based Zinc-Binding Sequences and the Antimicrobial Activity of Calprotectin. J. Infect. Dis. 1998, 177, 812–814. [Google Scholar] [CrossRef]
- Delaney, S.; Pascaly, M.; Bhattacharya, P.K.; Han, K.; Barton, J.K. Oxidative Damage by Ruthenium Complexes Containing the Dipyridophenazine Ligand or Its Derivatives: A Focus on Intercalation. Inorg. Chem. 2002, 41, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and Human Health: An Update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.d.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R.S. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Kozanecka-Okupnik, W.; Jasiewicz, B.; Pospieszny, T.; Jastrząb, R.; Skrobańska, M.; Mrówczyńska, L. Spectroscopy, Molecular Modeling and Anti-Oxidant Activity Studies on Novel Conjugates Containing Indole and Uracil Moiety. J. Mol. Struct. 2018, 1169, 130–137. [Google Scholar] [CrossRef]
- Kozanecka-Okupnik, W.; Sierakowska, A.; Berdzik, N.; Kowalczyk, I.; Mrówczyńska, L.; Jasiewicz, B. New Triazole-Bearing Gramine Derivatives—Synthesis, Structural Analysis and Protective Effect against Oxidative Haemolysis. Nat. Prod. Res. 2022, 36, 3413–3419. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.-H. Reactive Oxygen Species: From Health to Disease. Swiss. Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Pietraszek, P.; Walczak, P. Characteristics and possible applications of bacteria of the genus Bacillus isolated from the soil. Pol. J. Agric. 2014, 16, 37–44. [Google Scholar]
- Zhu, M.; Zhu, Q.; Yang, Z.; Liang, Z. Clinical Characteristics of Patients with Micrococcus luteus Bloodstream Infection in a Chinese Tertiary-Care Hospital. Pol. J. Microbiol. 2021, 70, 321–326. [Google Scholar] [CrossRef]
- Necel, A.; Bloch, S.; Topka-Bielecka, G.; Janiszewska, A.; Łukasiak, A.; Nejman-Faleńczyk, B.; Węgrzyn, G. Synergistic Effects of Bacteriophage vB_Eco4-M7 and Selected Antibiotics on the Biofilm Formed by Shiga Toxin-Producing Escherichia coli. Antibiotics 2022, 11, 712. [Google Scholar] [CrossRef]
- Wołejko, E.; Wydro, U.; Jabłońska-Trypuć, A.; Butarewicz, A.; Łoboda, T. Pseudomonas fluoresces occurrence in soil after fertilization with sewage sludge. Ekon. I Sr.—Econ. Environ. 2018, 65, 195–204. [Google Scholar]
- Wenderoth, M.; Garganese, F.; Schmidt-Heydt, M.; Soukup, S.T.; Ippolito, A.; Sanzani, S.M.; Fischer, R. Alternariol as virulence and colonization factor of Alternaria alternata during plant infection. Mol. Microbiol. 2019, 112, 131–146. [Google Scholar] [CrossRef]
- Pastuszak, J.; Szczerba, A.; Dziurka, M.; Hornyák, M.; Kopeć, P.; Szklarczyk, M.; Płażek, A. Physiological and Biochemical Response to Fusarium culmorum Infection in Three Durum Wheat Genotypes at Seedling and Full Anthesis Stage. Int. J. Mol. Sci. 2021, 22, 7433. [Google Scholar] [CrossRef]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The Destructive Fungal Pathogen Botrytis cinerea—Insights from Genes Studied with Mutant Analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef]
- Mironenka, J.; Różalska, S.; Bernat, P. Potential of Trichoderma harzianum and Its Metabolites to Protect Wheat Seedlings against Fusarium culmorum and 2,4-D. Int. J. Mol. Sci. 2021, 22, 13058. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, Version 1.171.37.33; Agilent Technologies Ltd.: Yarnton, UK, 2014.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyńska, L.; Hagerstrand, H. Platelet-activating factor interaction with the human erythrocyte membrane. J. Biochem. Mol. Toxicol. 2009, 23, 345–348. [Google Scholar] [CrossRef] [PubMed]





 | ||||
| φ1 | φ2 | φ3 | ||
| 1 * | R=H, R’=H | 86.7 (4) | −79.1 (4) | −46.0 (4) |
| 2 * | R=Me, R’=H | 97.5 (3) | −87.2 (3) | −47.0 (3) |
| 3 | R=iPr R’=H | 88.9 (5) | −14.5 (7) | 59.9 (6) |
| 71.9 (5) | 18.1 (6) | 54.7 (6) | ||
| L3 | R=iPr, R’=H | −65.39 (17) | −34.9 (2) | |
| 4 * | R=Et, R’=Me | 81.6 (8) | 59.2 (7) | −66.8 (7) |
| 5 | R=Ph, R’=H | 68.7 (5) | 41.8 (5) | −49.0 (5) |
| 68.1 (6) | 46.1 (6) | −41.6 (6) | ||
| L5 | R=Ph, R’=H | −62.47 (19) | −31.3 (2) | |
| D—H···A | D—H (Å) | H···A (Å) | D···A (Å) | D—H···A (°) |
|---|---|---|---|---|
| 1 | ||||
| N1—H1···Cl1 i | 0.86 | 2.45 | 3.260 (3) | 157 |
| 2 | ||||
| N1—H1···Cl1 i | 0.86 | 2.58 | 3.345 (2) | 148 |
| 3 | ||||
| N1—H1···Cl1 ii | 0.86 | 2.47 | 3.324 (3) | 170 |
| N21—H21···Cl2 iii | 0.86 | 2.68 | 3.398 (4) | 142 |
| 4 | ||||
| N1—H1···Cl1 iv | 0.86 | 2.57 | 3.373 (7) | 156 |
| 5 | ||||
| N1—H1···Cl2 v | 0.86 | 2.49 | 3.337 (3) | 167 |
| N21—H21···Cl1 vi | 0.86 | 2.40 | 3.236 (3) | 165 |
| Complex | Zone of Growth Inhibition [mm] | |||
|---|---|---|---|---|
| Micrococus luteus | Bacillus subtilis | Escherichia coli | Pseudomonas fluorescens | |
| 1 | 10.6 | 4 | 5 | 3 |
| 2 | 6 | 4 | 4.3 | 3.4 |
| 3 | 3.7 | 3 | 3.5 | 0 |
| 4 | 2.4 | 2.3 | 3.7 | 3.3 |
| 5 | 8 | 4 | 5 | 4 |
| 6 | 7.6 | 4.8 | 4 | 4 |
| Complex | Zone of Growth Inhibition [mm] | Zones of Growth Stimulation [mm] | |||
|---|---|---|---|---|---|
| Alternaria alternata | Fusarium culmorum | Trichoderma harzianum | Trichoderma atroviride | Botrysis cinera | |
| 1 | 4.5 | 0 | 10 | 0 | 13.3 |
| 2 | 7.5 | 2 | 3 | 4 | 16.8 |
| 3 | 4.5 | 6 | 10 | 2 | 17 |
| 4 | 2.5 | 0 | 11.5 | 2 | 21.2 |
| 5 | 10 | 6.4 | 9.8 | 2 | 15.3 |
| 6 | 7.5 | 9.2 | 11.5 | 10 | 11.7 |
| Compound | LogP | GI Absorption | BBB Permeant | LogS | Solubility Class * |
|---|---|---|---|---|---|
| 1 | 2.91 | High | Yes | −7.16 | Poorly soluble |
| 2 | 3.48 | High | Yes | −7.89 | Poorly soluble |
| 3 | 4.52 | Low | No | −9.03 | Poorly soluble |
| 4 | 4.57 | High | No | −9.38 | Poorly soluble |
| 5 | 5.33 | Low | No | −10.9 | Insoluble |
| 6 | 4.79 | Low | No | −10.03 | Insoluble |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Chemical formula | C24H22Cl2N6Zn | C26H26Cl2N6Zn | C30H34Cl2N6Zn | C30H34Cl2N6Zn | C36H30Cl2N6Zn |
| Mr | 530.74 | 558.80 | 614.90 | 614.90 | 682.93 |
| Crystal system, space group | Orthorhombic, Fdd2 | Orthorhombic, Fdd2 | Triclinic, P | Monoclinic, C2/c | Monoclinic, P21/c |
| a (Å) b (Å) c (Å) | 25.6543 (9) 18.1710 (6) 10.4135 (3) | 30.2233 (5) 16.0385 (2) 10.5687 (1) | 8.6293 (5) 12.5629 (7) 15.3951 (13) | 21.6193 (4) 9.0501 (2) 15.1471 (3) | 16.9185 (9) 10.2496 (3) 19.3748 (6) |
| α (°) β (°) γ (°) | 90 90 90 | 90 90 90 | 78.332 (6) 76.354 (6) 71.776 (5) | 90 93.383 (2) 90 | 90 105.642 (4) 90 |
| V (Å3) | 4854.4 (3) | 5123.03 (12) | 1525.51 (19) | 2958.47 (10) | 3235.3 (2) |
| Z | 8 | 8 | 2 | 4 | 4 |
| Dx (Mg m−3) | 1.452 | 1.449 | 1.339 | 1.381 | 1.402 |
| μ (mm−1) | 1.26 | 1.19 | 1.01 | 1.04 | 0.96 |
| Crystal size (mm) | 0.25 × 0.18 × 0.15 | 0.60 × 0.20 × 0.12 | 0.40 × 0.25 × 0.25 | 0.35 × 0.35 × 0.05 | 0.35 × 0.23 × 0.03 |
| Data collection | |||||
| Tmin, Tmax | 0.955, 1.000 | 0.901, 1.000 | 0.973, 1.000 | 0.830, 1.000 | 0.962, 1.000 |
| No. of measured, independent and observed [I > 2θ(I)] reflections | 18,213, 2834, 2425 | 19,144, 2957, 2773 | 14,779, 14,779, 7369 | 39,542, 3485, 2193 | 38,365, 7181, 3683 |
| Rint | 0.024 | 0.017 | – | 0.035 | 0.063 |
| (sin θ/λ)max (Å−1) | 0.670 | 0.672 | 0.674 | 0.667 | 0.667 |
| Refinement | |||||
| R[F2 > 2σ(F2)], wR(F2), S | 0.028, 0.062, 1.03 | 0.021, 0.057, 1.09 | 0.046, 0.123, 0.87 | 0.077, 0.239, 1.03 | 0.063, 0.135, 1.02 |
| No. of reflections | 2834 | 2957 | 14,779 | 3485 | 7181 |
| No. of parameters | 150 | 160 | 357 | 179 | 406 |
| No. of restraints | 1 | 1 | 36 | 30 | 12 |
| Δρmax, Δρmin (e Å−3) | 0.21, −0.17 | 0.24, −0.15 | 0.59, −0.32 | 1.04, −0.39 | 0.63, −0.32 |
| Absolute structure parameter | 0.003 (4) | −0.013 (3) | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babijczuk, K.; Warżajtis, B.; Starzyk, J.; Mrówczyńska, L.; Jasiewicz, B.; Rychlewska, U. Synthesis, Structure and Biological Activity of Indole–Imidazole Complexes with ZnCl2: Can Coordination Enhance the Functionality of Bioactive Ligands? Molecules 2023, 28, 4132. https://doi.org/10.3390/molecules28104132
Babijczuk K, Warżajtis B, Starzyk J, Mrówczyńska L, Jasiewicz B, Rychlewska U. Synthesis, Structure and Biological Activity of Indole–Imidazole Complexes with ZnCl2: Can Coordination Enhance the Functionality of Bioactive Ligands? Molecules. 2023; 28(10):4132. https://doi.org/10.3390/molecules28104132
Chicago/Turabian StyleBabijczuk, Karolina, Beata Warżajtis, Justyna Starzyk, Lucyna Mrówczyńska, Beata Jasiewicz, and Urszula Rychlewska. 2023. "Synthesis, Structure and Biological Activity of Indole–Imidazole Complexes with ZnCl2: Can Coordination Enhance the Functionality of Bioactive Ligands?" Molecules 28, no. 10: 4132. https://doi.org/10.3390/molecules28104132
APA StyleBabijczuk, K., Warżajtis, B., Starzyk, J., Mrówczyńska, L., Jasiewicz, B., & Rychlewska, U. (2023). Synthesis, Structure and Biological Activity of Indole–Imidazole Complexes with ZnCl2: Can Coordination Enhance the Functionality of Bioactive Ligands? Molecules, 28(10), 4132. https://doi.org/10.3390/molecules28104132