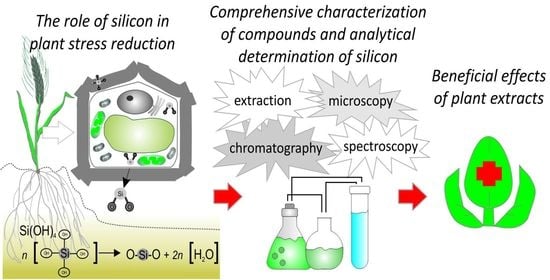
Comprehensive Study of Si-Based Compounds in Selected Plants (Pisum sativum L., Medicago sativa L., Triticum aestivum L.)
Abstract
:1. Introduction
2. Silicon in Soil

3. Silicon Uptake by Plants
4. Silicon Role in Plant Stress Alleviation
5. Si-Mediated Alleviation of Stress Effects in Wheat
6. Si-Mediated Alleviation of Stress Effects in Pea Plants
7. Si-Mediated Alleviation of Stress Effects in Alfalfa Plants
8. Extraction Methods to Assess the Presence of Silicon in Plants
9. Sample Preparation and Si Concentration in Plant Material
10. Extraction Methods to Assess the Presence of Plant Biologically Active Compounds
10.1. Maceration
10.2. Pre-Treatment
10.3. Extraction
10.4. Purification, Quantification
10.5. Derivatization
10.6. Accelerated Solvent Extraction (ASE)
10.7. Supercritical Fluid Extraction (SFE)
10.8. Microwave Assisted Extraction (MAE)
11. Techniques Applied in the Determination of Silicon in Plants
11.1. Direct Techniques
11.1.1. XRF
11.1.2. Laser Ablation
11.1.3. Laser-Induced Breakdown
11.1.4. Electrothermal Vaporization
11.1.5. EDX
11.2. Indirect Techniques
11.2.1. ICP-OES
11.2.2. ICP-MS
11.2.3. Colorimetric Method
11.2.4. Silicon Speciation
11.2.5. Organic Silicon Compounds in Plants
11.2.6. Microscopy (SEM, TEM, AFM, Light Microscopy) Studies of Silicon in Plants
11.2.7. Structural Analysis (IR and Raman) of Silicon in Plants
11.3. Analytical Techniques for the Separation and Isolation of Biologically Active Compounds
11.3.1. Thin-Layer Chromatography (TLC)
11.3.2. High Performance Liquid Chromatography (HPLC)
11.3.3. Gas Chromatography (GC)
12. Antimicrobial Properties of Medicago spp., P. sativum, and Triticum spp.
13. The Effect of Medicago spp., P. sativum, and Triticum spp. on Eukaryotic Cells
13.1. The Antitumor Activity of Medicago spp., P. sativum, and Triticum spp.
13.2. The Influence of Medicago spp., P. sativum, and Triticum spp. on Normal Eukaryotic Cells
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| ASE | Accelerated Solvent Extraction |
| AID | Autoclave Induced Digestion |
| BEC | Background Equivalent Concentration |
| DART | Direct Analysis in Real-Time |
| ETV | Electrothermal Vaporization |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GAE | Gallic Acid Equivalents |
| HPTLC | High-Performance Thin-Layer Chromatography |
| HRE | Heat Reflux Extraction |
| HSAB | Hard and Soft Acids and Base Theory |
| ICP | Inductively Coupled Plasma |
| IC50 | half maximal inhibitory concentration |
| LA | Laser Ablation |
| LIBS | Laser-Induced Breakdown Spectrometry |
| MIC | Minimum Inhibitory Concentration |
| MALDI MS | Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry |
| MBC | Molybdenum Blue Colorimetry |
| MAE | Microwave Assisted Extraction |
| NMR | Nuclear magnetic resonance |
| NIRS | Near Infrared Reflectance Spectroscopy |
| OPLC | Overpressure-Layer Chromatography |
| OES | Optical Emission Spectrometry |
| OID | Oven Induced Digestion |
| PEC | Planar Electro-Chromatography |
| RF | Radio Frequency |
| SFE | Supercritical Fluid Extraction |
| SEM-EDX | Scanning Electron Microscopy with Energy Dispersive X-ray Detector |
| SEC | Size Exclusion Chromatography |
| TLC-DB | Thin-Layer Chromatography-Direct Bioautography |
| TEM | Transmission Electron Microscopy |
| UAE | Ultrasound-Assisted Extraction |
| XRF | X-ray Fluorescence |
References
- Basu, S.; Kumar, G. Exploring the significant contribution of silicon in regulation of cellular redox homeostasis for conferring stress tolerance in plants. Plant Physiol. Biochem. 2021, 166, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Matichenkov, V.V.; Bocharnikova, E.A. Chapter 13 The relationship between silicon and soil physical and chemical properties. In Silicon in Agriculture; Elsevier: Amsterdam, The Netherlands, 2001; pp. 209–219. ISBN 9780444502629. [Google Scholar]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Biochem. 2021, 165, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420093704. [Google Scholar]
- Georgieva-Krasteva, L.; Hristova, I.; Mihaylova, D.; Dobreva, K. Spelt (Triticum aestivum ssp. Spelta)—from Field to Cosmetics. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 613–617. [Google Scholar] [CrossRef]
- Rasul, M.G. Extraction, Isolation and Characterization of Natural Products from Medicinal Plants. Int. J. Basic Sci. Appl. Comput. 2018, 2, F0076122618. [Google Scholar]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Walczak-Skierska, J.; Możeński, C.; Buszewski, B. Extraction and Determination of Polar Bioactive Compounds from Alfalfa (Medicago sativa L.) Using Supercritical Techniques. Molecules 2019, 24, 4608. [Google Scholar] [CrossRef]
- Waksmundzka-Hajnos, M.; Sherma, J.; Kowalska, T. Thin Layer Chromatography in Phytochemistry; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9781420046786. [Google Scholar]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, Isolation And Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Trad. Compl. Alt. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Gong, J.H.; Randall, P.D.; Flowers, J.T. Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ. 2006, 29, 1970–1979. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Savant, N.K.; Korndörfer, G.H.; Datnoff, L.E.; Snyder, G.H. Silicon nutrition and sugarcane production: A review. J. Plant Nutr. 1999, 22, 1853–1903. [Google Scholar] [CrossRef]
- Sauer, D.; Saccone, L.; Conley, D.J.; Herrmann, L.; Sommer, M. Review of methodologies for extracting plant-available and amorphous Si from soils and aquatic sediments. Biogeochemistry 2006, 80, 89–108. [Google Scholar] [CrossRef]
- Drees, R.L.; Wilding, P.L.; Smeck, E.N.; Senkayi, L.A. Silica in Soils: Quartz and Disordered Silica Polymorphs. In Minerals in Soil Environments; Dixon, J., Weed, S., Eds.; SSSA: Madison, Wisconsin, 1989; pp. 913–974. ISBN 9780891188605. [Google Scholar]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef]
- Santi, L.P.; Haris, N.; Mulyanto, D. Effect of bio-silica on drought tolerance in plants. IOP Conf. Ser. Earth Environ. Sci. 2018, 183, 12014. [Google Scholar] [CrossRef]
- Matichenkov, V.; Bocharnikova, E.A.; Calvert, D.V.; Snyder, G.H. Comparison study of soil silicon status in sandy soils of south Florida. Soil Crop Sci. Soc. Fla. Proc. 2000, 59, 132–137. [Google Scholar]
- Ehrlich, H.; Demadis, K.D.; Pokrovsky, O.S.; Koutsoukos, P.G. Modern Views on Desilicification: Biosilica and Abiotic Silica Dissolution in Natural and Artificial Environments. Chem. Rev. 2010, 110, 4656–4689. [Google Scholar] [CrossRef]
- Bienert, G.P.; Thorsen, M.; Schüssler, M.D.; Nilsson, H.R.; Wagner, A.; Tamás, M.J.; Jahn, T.P. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008, 6, 26. [Google Scholar] [CrossRef]
- Marschner, H. Function of Mineral Nutrients: Micronutrients. In Mineral Nutrition of Higher Plants, 2nd ed.; Marschner, H., Ed.; Academic Press: Amsterdam, The Netherlands, 1995; ISBN 9780080571874. [Google Scholar]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef]
- Perry, C.C.; Lu, Y. Preparation of silicas from silicon complexes: Role of cellulose in polymerisation and aggregation control. Faraday Trans. 1992, 88, 2915–2921. [Google Scholar] [CrossRef]
- Duboc, O.; Robbe, A.; Santner, J.; Folegnani, G.; Gallais, P.; Lecanuet, C.; Zehetner, F.; Nagl, P.; Wenzel, W.W. Silicon Availability from Chemically Diverse Fertilizers and Secondary Raw Materials. Environ. Sci. Technol. 2019, 53, 5359–5368. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delvaux, B. Phytolith-rich biochar: A potential Si fertilizer in desilicated soils. GCB Bioenergy 2019, 11, 1264–1282. [Google Scholar] [CrossRef]
- Huang, C.; Wang, L.; Gong, X.; Huang, Z.; Zhou, M.; Li, J.; Wu, J.; Chang, S.X.; Jiang, P. Silicon fertilizer and biochar effects on plant and soil PhytOC concentration and soil PhytOC stability and fractionation in subtropical bamboo plantations. Sci. Total Environ. 2020, 715, 136846. [Google Scholar] [CrossRef]
- Xiang, T.; Ying, Y.; Teng, J.; Huang, Z.; Wu, J.; Meng, C.; Jiang, P.; Tang, C.; Li, J.; Zheng, R. Sympodial bamboo species differ in carbon bio-sequestration and stocks within phytoliths of leaf litters and living leaves. Environ. Sci. Pollut. Res. 2016, 23, 19257–19265. [Google Scholar] [CrossRef]
- Liang, Y.; Liao, M.; Fang, Z.; Guo, J.; Xie, X.; Xu, C. How silicon fertilizer improves nitrogen and phosphorus nutrient availability in paddy soil? J. Zhejiang Univ. Sci. B 2021, 22, 521–532. [Google Scholar] [CrossRef]
- Takahashi, E.; Ma, J.F.; Miyake, Y. The possibility of silicon as an essential element for higher plants. J. Agric. Food Chem. 1990, 2, 99–122. [Google Scholar]
- Savant, N.K.; Snyder, G.H.S.; Datnoff, L.E. Silicon Management and Sustainable Rice Production. Adv. Agron. 1996, 58, 151–199. [Google Scholar]
- Marron, A.O.; Ratcliffe, S.; Wheeler, G.L.; Goldstein, R.E.; King, N.; Not, F.; de Vargas, C.; Richter, D.J. The Evolution of Silicon Transport in Eukaryotes. Mol. Biol. Evol. 2016, 33, 3226–3248. [Google Scholar] [CrossRef]
- Ma, J.F. Silicon transporters in higher plants. Adv. Exp. Med. Biol. 2010, 679, 99–109. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Ichii, M.; Wu, G.F. A Rice Mutant Defective in Si Uptake. Plant Physiol. 2002, 130, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Ma, J.F.; Iwashita, T. Identification of the Silicon Form in Xylem Sap of Rice (Oryza sativa L.). Plant Cell Physiol. 2005, 46, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Marafon, A.C.; Endres, L. Silicon: Fertilization and nutrition in higher plants. RCA 2013, 56, 380–388. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Bala, M.; Pareek, A.; Singla-Pareek, S.L.; Singh, A.K. Silicon-mediated abiotic and biotic stress mitigation in plants: Underlying mechanisms and potential for stress resilient agriculture. Plant Physiol. Biochem. 2021, 163, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Blackman, E. Observations on the development of the silica cells of the leaf sheath of wheat (Triticum aestivum). Can. J. Bot. 1969, 47, 827–838. [Google Scholar] [CrossRef]
- Dietrich, D.; Hinke, S.; Baumann, W.; Fehlhaber, R.; Bäucker, E.; Rühle, G.; Wienhaus, O.; Marx, G. Silica accumulation in Triticum aestivum L. and Dactylis glomerata L. Anal. Bioanal. Chem. 2003, 376, 399–404. [Google Scholar] [CrossRef]
- Meunier, J.D.; Barboni, D.; Anwar-ul-Haq, M.; Levard, C.; Chaurand, P.; Vidal, V.; Grauby, O.; Huc, R.; Laffont-Schwob, I.; Rabier, J.; et al. Effect of phytoliths for mitigating water stress in durum wheat. New Phytol. 2017, 215, 229–239. [Google Scholar] [CrossRef]
- Bennett, M.D. Silicon Deposition in the Roots of Hordeum sativum Jess, Avena sativa L. and Triticum aestivum L. Ann. Bot. 1982, 50, 239–245. [Google Scholar] [CrossRef]
- Hodson, M.J.; Sangster, A.G. Subcellular localization of mineral deposits in the roots of wheat (Triticum aestivum L.). Protoplasma 1989, 151, 19–32. [Google Scholar] [CrossRef]
- Casey, H.W.; Kinrade, D.S.; Knight, G.C.T.; Rains, W.D.; Epstein, E. Aqueous silicate complexes in wheat, Triticum aestivum L. Plant Cell Environ. 2004, 27, 51–54. [Google Scholar] [CrossRef]
- Howladar, S.M.; Al-Robai, S.A.; Al-Zahrani, F.S.; Howladar, M.M.; Aldhebiani, A.Y. Silicon and its application method effects on modulation of cadmium stress responses in Triticum aestivum (L.) through improving the antioxidative defense system and polyamine gene expression. Ecotoxicol. Environ. Saf. 2018, 159, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yang, S.; Han, D.; Zhou, Z.; Li, X.; Liu, Y.; Zhang, B. Silicon alleviates cadmium toxicity in wheat seedlings (Triticum aestivum L.) by reducing cadmium ion uptake and enhancing antioxidative capacity. Environ. Sci. Pollut. Res. 2018, 25, 7638–7646. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Xuebin, Q.; Zhao, Z.; Du, Z.; Imtiaz, M.; Mehmood, F.; Hongfei, L.; Hussain, B.; Ashraf, M.N. Alleviatory effects of Silicon on the morphology, physiology, and antioxidative mechanisms of wheat (Triticum aestivum L.) roots under cadmium stress in acidic nutrient solutions. Sci. Rep. 2021, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Meunier, J.-D.; Miche, H.; Keller, C. Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J. Hazard. Mater. 2012, 209–210, 326–334. [Google Scholar] [CrossRef]
- Greger, M.; Kabir, A.H.; Landberg, T.; Maity, P.J.; Lindberg, S. Silicate reduces cadmium uptake into cells of wheat. Environ. Pollut. 2016, 211, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Thind, S.; Hussain, I.; Ali, S.; Rasheed, R.; Ashraf, M.A. Silicon Application Modulates Growth, Physio-Chemicals, and Antioxidants in Wheat (Triticum aestivum L.) Exposed to Different Cadmium Regimes. Dose-Response 2021, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Geilfus, C.-M.; Pitann, B.; Mühling, K.-H. Silicon-enhanced oxalate exudation contributes to alleviation of cadmium toxicity in wheat. Environ. Exp. Bot. 2016, 131, 10–18. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Xuebin, Q.; Yasin, G.; Cheng, H.; Mehmood, F.; Zain, M.; Shehzad, M.; Ahmad, M.I.; Riaz, L.; Rahim, A.; et al. Role of silicon on root morphological characters of wheat (Triticum aestivum L.) plants grown under Cd-contaminated nutrient solution. Acta Physiol. Plant 2021, 43, 60. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Pei, Z.F.; Ming, D.F.; Liu, D.; Wan, G.L.; Geng, X.X.; Gong, H.J.; Zhou, W.J. Silicon Improves the Tolerance to Water-Deficit Stress Induced by Polyethylene Glycol in Wheat (Triticum aestivum L.) Seedlings. J. Plant Growth Regul. 2010, 29, 106–115. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Kuşvuran, S.; Rady, M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.T.; Rodrigues, F.Á.; Oliveira, J.R.; Pereira, S.C.; Andrade, C.C.L.; Silveira, P.R.; Conceição, M.M. Wheat Resistance to Bacterial Leaf Streak Mediated by Silicon. J. Phytopathol. 2010, 158, 253–262. [Google Scholar] [CrossRef]
- Goussain, M.M.; Prado, E.; Moraes, J.C. Effect of silicon applied to wheat plants on the biology and probing behaviour of the greenbug Schizaphis graminum (Rond.) (Hemiptera: Aphididae). Neotrop. Entomol. 2005, 34, 807–813. [Google Scholar] [CrossRef]
- Parry, W.D.; Winslow, A. Electron-probe Microanalysis of Silicon Accumulation in the Leaves and Tendrils of Pisum sativum (L.) Following Root Severance. Ann. Bot. 1977, 41, 275–278. [Google Scholar] [CrossRef]
- Merwad, A.-R.M.A. Response of yield and nutrients uptake of pea plants to silicate under sandy soil conditions. Commun. Soil Sci. Plant Anal. 2018, 49, 1553–1562. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.F.; Ghosal, A.; Alam, M.F.; Kabir, A.H. Remediation of cadmium toxicity in field peas (Pisum sativum L.) through exogenous silicon. Ecotoxicol. Environ. Saf. 2017, 135, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Siddique, K.H.; Ahmad, P. Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol. 2018, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, X.; Guo, S.; Chen, X.; Chen, T.; He, Y.; Shabala, S.; Yu, M. Extracellular silica nanocoat formed by layer-by-layer (LBL) self-assembly confers aluminum resistance in root border cells of pea (Pisum sativum). J. Nanobiotechnol. 2019, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Arafa, S.A.; Attia, K.A.; Niedbała, G.; Piekutowska, M.; Alamery, S.; Abdelaal, K.; Alateeq, T.K.; Ali, M.A.M.; Elkelish, A.; Attallah, S.Y. Seed Priming Boost Adaptation in Pea Plants under Drought Stress. Plants 2021, 10, 2201. [Google Scholar] [CrossRef]
- Dann, E.K.; Muir, S. Peas grown in media with elevated plant-available silicon levels have higher activities of chitinase and β-1,3-glucanase, are less susceptible to a fungal leaf spot pathogen and accumulate more foliar silicon. Australas. Plant Pathol. 2002, 31, 9–13. [Google Scholar] [CrossRef]
- Głowacka, K.; Szultka-Młyńska, M.; Cichorek, M.; Orzoł, A.; Rogowska, A.; Cruzado Tafur, E.; Pomastowski, P.; Górecki, R.; Buszewski, B. Znaczenie krzemu dla wybranych gatunków roślin. Kosmos 2022, 71, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sahebi, M.; Hanafi, M.M.; Akmar, A.S.N.; Rafii, M.Y.; Azizi, P.; Tengoua, F.F.; Azwa, J.N.M.; Shabanimofrad, M. Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res. Int. 2015, 2015, 396010. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, Z.G. Forage Yield and Water Use Efficiency of Alfalfa Applied with Silicon under Water Deficit Conditions. Philipp. Agric. 2013, 96, 370–376. [Google Scholar]
- Kabir, A.H.; Hossain, M.M.; Khatun, M.A.; Mandal, A.; Haider, S.A. Role of Silicon Counteracting Cadmium Toxicity in Alfalfa (Medicago sativa L.). Front. Plant Sci. 2016, 7, 17. [Google Scholar] [CrossRef]
- Liu, D.; Liu, M.; Liu, X.-L.; Cheng, X.-G.; Liang, Z.-W. Silicon Priming Created an Enhanced Tolerance in Alfalfa (Medicago sativa L.) Seedlings in Response to High Alkaline Stress. Front. Plant Sci. 2018, 9, 716. [Google Scholar] [CrossRef]
- Meng, Y.; Yin, Q.; Yan, Z.; Wang, Y.; Niu, J.; Zhang, J.; Fan, K. Exogenous Silicon Enhanced Salt Resistance by Maintaining K+/Na+ Homeostasis and Antioxidant Performance in Alfalfa Leaves. Front. Plant Sci. 2020, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Carol, P.; Mouradi, M.; Savoure, A.; Farissi, M. Silicon improves physiological, biochemical, and morphological adaptations of alfalfa (Medicago sativa L.) during salinity stress. Symbiosis 2021, 85, 305–324. [Google Scholar] [CrossRef]
- Namieśnik, J.; Szefer, P. Preparing samples for analysis—The key to analytical success. Ecol. Chem. Eng. S-Chem. I Inz. Ekol. S 2008, 15, 167–244. [Google Scholar]
- Reidinger, S.; Ramsey, M.H.; Hartley, S.E. Rapid and accurate analyses of silicon and phosphorus in plants using a portable X-ray fluorescence spectrometer. New Phytol. 2012, 195, 699–706. [Google Scholar] [CrossRef]
- Masson, P.; Dalix, T.; Bussière, S. Determination of Major and Trace Elements in Plant Samples by Inductively Coupled Plasma–Mass Spectrometry. Commun. Soil Sci. Plant Anal. 2010, 41, 231–243. [Google Scholar] [CrossRef]
- Li, Z.; Song, Z.; Singh, B.P.; Wang, H. The impact of crop residue biochars on silicon and nutrient cycles in croplands. Sci. Total Environ. 2019, 659, 673–680. [Google Scholar] [CrossRef]
- Nakamura, R.; Cornelis, J.-T.; de Tombeur, F.; Nakagawa, M.; Kitajima, K. Comparative analysis of borate fusion versus sodium carbonate extraction for quantification of silicon contents in plants. J. Plant Res. 2020, 133, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Puppe, D.; Höhn, A.; Kaczorek, D.; Wanner, M.; Wehrhan, M.; Sommer, M. How big is the influence of biogenic silicon pools on short-term changes in water-soluble silicon in soils? Implications from a study of a 10-year-old soil–plant system. Biogeosciences 2017, 14, 5239–5252. [Google Scholar] [CrossRef]
- Barros, J.A.V.A.; de Souza, P.F.; Schiavo, D.; Nóbrega, J.A. Microwave-assisted digestion using diluted acid and base solutions for plant analysis by ICP OES. J. Anal. At. Spectrom. 2016, 31, 337–343. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Determination of the silicon concentration in plant material using Tiron extraction. New Phytol. 2010, 188, 902–906. [Google Scholar] [CrossRef]
- Clymans, W.; Conley, D.J.; Battles, J.J.; Frings, P.J.; Koppers, M.M.; Likens, G.E.; Johnson, C.E. Silica uptake and release in live and decaying biomass in a northern hardwood forest. Ecology 2016, 97, 3044–3057. [Google Scholar] [CrossRef]
- Snyder, G.H. Chapter 11 Methods for silicon analysis in plants, soils, and fertilizers. In Silicon in Agriculture; Elsevier: Amsterdam, The Netherlands, 2001; pp. 185–196. ISBN 9780444502629. [Google Scholar]
- Smis, A.; Ancin Murguzur, F.J.; Struyf, E.; Soininen, E.M.; Herranz Jusdado, J.G.; Meire, P.; Bråthen, K.A. Determination of plant silicon content with near infrared reflectance spectroscopy. Front. Plant Sci. 2014, 5, 496. [Google Scholar] [CrossRef]
- Taber, H.G.; Shogren, D.; Lu, G. Extraction of silicon from plant tissue with dilute HCl and HF and measurement by modified inductive coupled argon plasma procedures. Commun. Soil Sci. Plant Anal. 2002, 33, 1661–1670. [Google Scholar] [CrossRef]
- Masson, P.; Dauthieu, M.; Trolard, F.; Denaix, L. Application of direct solid analysis of plant samples by electrothermal vaporization-inductively coupled plasma atomic emission spectrometry: Determination of Cd and Si for environmental purposes. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 224–230. [Google Scholar] [CrossRef]
- Babu, T.; Tubana, B.; Paye, W.; Kanke, Y.; Datnoff, L. Establishing Soil Silicon Test Procedure and Critical Silicon Level for Rice in Louisiana Soils. Commun. Soil Sci. Plant Anal. 2016, 47, 1578–1597. [Google Scholar] [CrossRef]
- Kraska, J.E.; Breitenbeck, G.A. Survey of the Silicon Status of Flooded Rice in Louisiana. Agron. J. 2010, 102, 523–529. [Google Scholar] [CrossRef]
- Paye, W.S. Silicon Fertilization in Rice and Wheat: Dynamics with Trace Elements and Effect of Silicate Slag Granular Size on the Release Pattern of Monosilicic Acid in Soil. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2019. [Google Scholar]
- Katz, O.; Puppe, D.; Kaczorek, D.; Prakash, N.B.; Schaller, J. Silicon in the Soil-Plant Continuum: Intricate Feedback Mechanisms within Ecosystems. Plants 2021, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Rimstidt, J.D. Solubility and dissolution rate of silica in acid fluoride solutions. Geochim. Cosmochim. Acta 2009, 73, 7045–7059. [Google Scholar] [CrossRef]
- Saito, K.; Yamamoto, A.; Sa, T.; Saigusa, M. Rapid, Micro-Methods to Estimate Plant Silicon Content by Dilute Hydrofluoric Acid Extraction and Spectrometric Molybdenum Method. Soil Sci. Plant Nutr. 2005, 51, 29–36. [Google Scholar] [CrossRef]
- Santos, M.A.; Silva, A.B.S.; Machado, R.C.; Dias, E.A.; Barros, J.A.; Nogueira, A.R.A. Silicon determination by microwave-induced plasma optical emission spectrometry: Considerations and strategies for the use of tetrafluorboric acid and sodium hydroxide in sample preparation procedures. Spectrochim. Acta Part B At. Spectrosc. 2020, 167, 105842. [Google Scholar] [CrossRef]
- He, C.; Ma, J.; Wang, L. A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytol. 2015, 206, 1051–1062. [Google Scholar] [CrossRef]
- Gholami, M.; Behkami, S.; Zain, S.M.; Bakirdere, S. A simple design for microwave assisted digestion vessel with low reagent consumption suitable for food and environmental samples. Sci. Rep. 2016, 6, 37186. [Google Scholar] [CrossRef]
- Xiao, Z.; Peng, M.; Mei, Y.; Tan, L.; Liang, Y. Effect of organosilicone and mineral silicon fertilizers on chemical forms of cadmium and lead in soil and their accumulation in rice. Environ. Pollut. 2021, 283, 117107. [Google Scholar] [CrossRef] [PubMed]
- Bokor, B.; Soukup, M.; Vaculík, M.; Vd’ačný, P.; Weidinger, M.; Lichtscheidl, I.; Vávrová, S.; Šoltys, K.; Sonah, H.; Deshmukh, R.; et al. Silicon Uptake and Localisation in Date Palm (Phoenix dactylifera)—A Unique Association with Sclerenchyma. Front. Plant Sci. 2019, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Kido, N.; Yokoyama, R.; Yamamoto, T.; Furukawa, J.; Iwai, H.; Satoh, S.; Nishitani, K. The matrix polysaccharide (1;3,1;4)-β-D-glucan is involved in silicon-dependent strengthening of rice cell wall. Plant Cell Physiol. 2015, 56, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Gianoncelli, A.; Kourousias, G.; Green, K.; McKenna, B.A. Alleviation of Al Toxicity by Si Is Associated with the Formation of Al–Si Complexes in Root Tissues of Sorghum. Front. Plant Sci. 2017, 8, 2189. [Google Scholar] [CrossRef]
- Bowen, J.E. Manganese-silicon interaction and its effect on growth of Sudan grass. Plant Soil 1972, 37, 577–588. [Google Scholar] [CrossRef]
- Pan, T.; Zhang, J.; He, L.; Hafeez, A.; Ning, C.; Cai, K. Silicon Enhances Plant Resistance of Rice against Submergence Stress. Plants 2021, 10, 767. [Google Scholar] [CrossRef]
- Raid, R.N.; Anderson, D.L.; Ulloa, M.F. Influence of cultivar and amendment of soil with calcium silicate slag on foliar disease development and yield of sugar-cane. Crop Prot. 1992, 11, 84–88. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Ojha, K.S.; Aznar, R.; O’Donnell, C.; Tiwari, B.K. Ultrasound technology for the extraction of biologically active molecules from plant, animal and marine sources. Trends Anal. Chem. 2020, 122, 115663. [Google Scholar] [CrossRef]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, purification and applications of curcumin from plant materials-A comprehensive review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Liu, X.; Wang, N.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; Ouyang, K.H.; et al. Investigation of Chemical Composition, Antioxidant Activity, and the Effects of Alfalfa Flavonoids on Growth Performance. Oxid. Med. Cell. Longev. 2020, 2020, 8569237. [Google Scholar] [CrossRef] [PubMed]
- Troszyńska, A.; Estrella, I.; López-Amóres, M.; Hernández, T. Antioxidant Activity of Pea (Pisum sativum L.) Seed Coat Acetone Extract. LWT-Food Sci. Technol. 2002, 35, 158–164. [Google Scholar] [CrossRef]
- Vatansever, S.; Xu, M.; Magallanes-López, A.; Chen, B.; Hall, C. Supercritical Carbon Dioxide + Ethanol Extraction to Improve Organoleptic Attributes of Pea Flour with Applications of Sensory Evaluation, HS-SPME-GC, and GC-Olfactory. Processes 2021, 9, 489. [Google Scholar] [CrossRef]
- Tambun, R.; Alexander, V.; Ginting, Y. Performance comparison of maceration method, soxhletation method, and microwave-assisted extraction in extracting active compounds from soursop leaves (Annona muricata): A review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1122, 012095. [Google Scholar] [CrossRef]
- Sadowska, B.; Budzyńska, A.; Więckowska-Szakiel, M.; Paszkiewicz, M.; Stochmal, A.; Moniuszko-Szajwaj, B.; Kowalczyk, M.; Różalska, B. New pharmacological properties of Medicago sativa and Saponaria officinalis saponin-rich fractions addressed to Candida albicans. J. Med. Microbiol. 2014, 63, 1076–1086. [Google Scholar] [CrossRef]
- Rafińska, K.; Pomastowski, P.; Wrona, O.; Górecki, R.; Buszewski, B. Medicago sativa as a source of secondary metabolites for agriculture and pharmaceutical industry. Phytochem. Lett. 2017, 20, 520–539. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Tava, A.; Mella, M.; Avato, P.; Biazzi, E.; Pecetti, L.; Bialy, Z.; Jurzysta, M. New Triterpenic Saponins from the Aerial Parts of Medicago arabica (L.) Huds. J. Agric. Food Chem. 2009, 57, 2826–2835. [Google Scholar] [CrossRef]
- Kiełbasa, A.; Krakowska-Sieprawska, A.; Kowalkowski, T.; Rafińska, K.; Buszewski, B. Distribution of sapogenins in morphological Medicago sativa L. parts: Comparison of various extraction techniques. J. Sep. Sci. 2020, 43, 671–680. [Google Scholar] [CrossRef]
- Khaledi, M.; Khaledi, F.; Asadi-Samani, M.; Gholipour, A.; Mahmoodi Kouhi, A. Phytochemical evaluation and antibacterial effects of Medicago sativa, Onosma sericeum, Parietaria judaica L., Phlomis persica and Echinophora platyloba DC. on Enterococcus faecalis. Biomed. Res. Ther. 2018, 5, 1941–1951. [Google Scholar] [CrossRef]
- Amber, R.; Adnan, M.; Tariq, A.; Khan, S.N.; Mussarat, S.; Hashem, A.; Al-huqail, A.A.; Al-Arjani, A.-B.F.; Abd_Allah, E.F. Antibacterial activity of selected medicinal plants of northwest Pakistan traditionally used against mastitis in livestock. Saudi J. Biol. Sci. 2018, 25, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Chegini, H.; Oshaghi, M.; Boshagh, M.A.; Foroutan, P.; Jahangiri, A.H. Antibacterial effect of Medicago sativa extract on the common bacterial in sinusitis infection. IJBMPH 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Golla, K.; Vutukuru, S.S.; Rani, J.U.; Meghanath, P.; Pasha, C. Screening of small peptides from various germinating seeds having antimicrobial activity. IOSR J. Pharm. Biol. Sci. 2016, 11, 52–60. [Google Scholar]
- Pagnussatt, F.A.; Bretanha, C.C.; Kupski, L.; Garda-Buffon, J.; Badiale-Furlong, E. Promising Antifungal Effect of Rice (Oryza sativa L.), Oat (Avena sativa L.) and Wheat (Triticum aestivum L.) Extracts. J. Appl. Biotechnol. Rep. 2013, 1, 37–44. [Google Scholar] [CrossRef]
- Rodrigues, F.; Palmeira-de-Oliveira, A.; das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B. Medicago spp. extracts as promising ingredients for skin care products. Ind. Crops Prod. 2013, 49, 634–644. [Google Scholar] [CrossRef]
- Sangwan, Y.S.; Hooda, T.; Kumar, H. Antibacterial, Antioxidant, Antimicrobial And Wound Healing Potential of Triticum aestivum & Terminalia bellirica. Int. J. Pharma Prof. Res. 2013, 4, 786–793. [Google Scholar]
- Hadrich, F.; Arbi, M.E.; Boukhris, M.; Sayadi, S.; Cherif, S. Valorization of the Peel of Pea: Pisum sativum by Evaluation of Its Antioxidant and Antimicrobial Activities. J. Oleo Sci. 2014, 63, 1177–1183. [Google Scholar] [CrossRef]
- Sundaresan, A.; Selvi, A.; Manonmani, H.K. The Anti-Microbial Properties of Triticum aestivum (Wheat Grass) Extract. Int. J. Biotech. Well. Indus. 2015, 4, 84–91. [Google Scholar] [CrossRef]
- Kim, M.-H.; Jo, S.-H.; Ha, K.-S.; Song, J.-H.; Jang, H.-D.; Kwon, Y.-I. Antimicrobial activities of 1,4-benzoquinones and wheat germ extract. J. Microbiol. Biotechnol. 2010, 20, 1204–1209. [Google Scholar] [CrossRef]
- Nair, S.S.; Madembil, N.C.; Nair, P.; Raman, S.; Veerabadrappa, S.B. Comparative analysis of the antibacterial activity of some phytolectins. Int. Curr. Pharm. J. 2013, 2, 18–22. [Google Scholar] [CrossRef]
- Narendhirakannan, R.T.; Nirmala, J.G.; Caroline, A.; Lincy, S.; Saj, M.; Durai, D. Evaluation of antibacterial, antioxidant and wound healing properties of seven traditional medicinal plants from India in experimental animals. Asian Pac. J. Trop. Biomed. 2012, 2, S1245–S1253. [Google Scholar] [CrossRef]
- Caunii, A.; Pribac, G.; Grozea, I.; Gaitin, D.; Samfira, I. Design of optimal solvent for extraction of bio-active ingredients from six varieties of Medicago sativa. Chem. Cent. J. 2012, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Bialy, Z.; Jurzysta, M.; Mella, M.; Tava, A. Triterpene saponins from aerial parts of Medicago arabica L. J. Agric. Food Chem. 2004, 52, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Bucci, R.; Tava, A.; Vitali, C.; Rosato, A.; Bialy, Z.; Jurzysta, M. Antimicrobial activity of saponins from Medicago sp.: Structure-activity relationship. Phytother. Res. 2006, 20, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Abbruscato, P.; Tosi, S.; Crispino, L.; Biazzi, E.; Menin, B.; Picco, A.M.; Pecetti, L.; Avato, P.; Tava, A. Triterpenoid Glycosides from Medicago sativa as Antifungal Agents against Pyricularia oryzae. J. Agric. Food Chem. 2014, 62, 11030–11036. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Da Machado, A.P.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Povilaitis, D.; Šulniūtė, V.; Venskutonis, P.R.; Kraujalienė, V. Antioxidant properties of wheat and rye bran extracts obtained by pressurized liquid extraction with different solvents. J. Cereal Sci. 2015, 62, 117–123. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Rabalski, I.; Bryan, M. Accelerated, Microwave-Assisted, and Conventional Solvent Extraction Methods Affect Anthocyanin Composition from Colored Grains. J. Food Sci. 2014, 79, C138–C146. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Chen, H.; Ji, A.; Qiu, S.; Liu, Y.; Zhu, Q.; Yin, L. Covalent conjugation of bovine serum album and sugar beet pectin through Maillard reaction/laccase catalysis to improve the emulsifying properties. Food Hydrocoll. 2018, 76, 173–183. [Google Scholar] [CrossRef]
- Krakowska, A.; Rafińska, K.; Walczak, J.; Kowalkowski, T.; Buszewski, B. Comparison of Various Extraction Techniques of Medicago sativa: Yield, Antioxidant Activity, and Content of Phytochemical Constituents. J. AOAC Int. 2017, 100, 1681–1693. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Analysis of vitamin K1 in fruits and vegetables using accelerated solvent extraction and liquid chromatography tandem mass spectrometry with atmospheric pressure chemical ionization. Food Chem. 2016, 192, 402–408. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Mulcahey, L.J.; Taylor, L.T. Supercritical fluid extraction. Microchim. Acta 1992, 108, 115–132. [Google Scholar] [CrossRef]
- Athukorala, Y.; Hosseinian, F.S.; Mazza, G. Extraction and fractionation of alkylresorcinols from triticale bran by two-step supercritical carbon dioxide. LWT-Food Sci. Technol. 2010, 43, 660–665. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Rafińska, K.; Walczak-Skierska, J.; Kiełbasa, A.; Buszewski, B. Promising Green Technology in Obtaining Functional Plant Preparations: Combined Enzyme-Assisted Supercritical Fluid Extraction of Flavonoids Isolation from Medicago Sativa Leaves. Materials 2021, 14, 2724. [Google Scholar] [CrossRef]
- Krakowska, A.; Rafińska, K.; Walczak, J.; Buszewski, B. Enzyme-assisted optimized supercritical fluid extraction to improve Medicago sativa polyphenolics isolation. Ind. Crops Prod. 2018, 124, 931–940. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.; Portugal, I.; Silva, C.M. Lupane-type triterpenoids from Acacia dealbata bark extracted by different methods. Ind. Crops Prod. 2021, 170, 113734. [Google Scholar] [CrossRef]
- Zaniol, F.; Calisto, J.F.; Cozzer, G.; Ferro, D.M.; Dias, J.L.; Rodrigues, L.G.; Mazzutti, S.; Rezende, R.S.; Simões, D.A.; Ferreira, S.R.; et al. Comparative larvicidal effect of Pterodon spp. extracts obtained by different extraction methods. J. Supercrit. Fluids 2020, 166, 104993. [Google Scholar] [CrossRef]
- Favareto, R.; Teixeira, M.B.; Soares, F.A.L.; Belisário, C.M.; Corazza, M.L.; Cardozo-Filho, L. Study of the supercritical extraction of Pterodon fruits (Fabaceae). J. Supercrit. Fluids 2017, 128, 159–165. [Google Scholar] [CrossRef]
- Bogdanovic, A.; Tadic, V.; Stamenic, M.; Petrovic, S.; Skala, D. Supercritical carbon dioxide extraction of Trigonella foenum-graecum L. seeds: Process optimization using response surface methodology. J. Supercrit. Fluids 2016, 107, 44–50. [Google Scholar] [CrossRef]
- Ge, Y.; Yan, H.; Hui, B.; Ni, Y.; Wang, S.; Cai, T. Extraction of Natural Vitamin E from Wheat Germ by Supercritical Carbon Dioxide. J. Agric. Food Chem. 2002, 50, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Rosa, A.; Falconieri, D.; Porcedda, S.; Dessì, M.; Marongiu, B. Extraction of Oil from Wheat Germ by Supercritical CO2. Molecules 2009, 14, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Oufnac, D.S.; Xu, Z.; Sun, T.; Sabliov, C.; Prinyawiwatkul, W.; Godber, J.S. Extraction of Antioxidants from Wheat Bran Using Conventional Solvent and Microwave-Assisted Methods. Cereal Chem. 2007, 84, 125–129. [Google Scholar] [CrossRef]
- Crundwell, F.K. On the Mechanism of the Dissolution of Quartz and Silica in Aqueous Solutions. ACS Omega 2017, 2, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.; Adame, A.; de Almeida, E.; Brasil, M.; Schaefer, C.; Krug, F. In situ Determination of K, Ca, S and Si in Fresh Sugar Cane Leaves by Handheld Energy Dispersive X-Ray Fluorescence Spectrometry. J. Braz. Chem. Soc. 2018, 29, 1086–1093. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J.M. The Effect of Silicon on Osmotic and Drought Stress Tolerance in Wheat Landraces. Plants 2021, 10, 814. [Google Scholar] [CrossRef]
- van der Ent, A.; Casey, L.W.; Blamey, F.P.C.; Kopittke, P.M. Time-resolved laboratory micro-X-ray fluorescence reveals silicon distribution in relation to manganese toxicity in soybean and sunflower. Ann. Bot. 2020, 126, 331–341. [Google Scholar] [CrossRef]
- Michel, B.E. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 1983, 72, 66–70. [Google Scholar] [CrossRef]
- Johnson, J. Accurate Measurements of Low Z Elements in Sediments and Archaeological Ceramics Using Portable X-ray Fluorescence (PXRF). J. Archaeol. Method Theory 2014, 21, 563–588. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Vivancos, J.; Guérin, V.; Sonah, H.; Labbé, C.; Belzile, F.; Bélanger, R.R. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol. Biol. 2013, 83, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, R.; Rybak, A.; Baranowska, I. Speciation analysis of elements in soil samples by XRF. Pol. J. Environ. Stud. 2002, 11, 473–482. [Google Scholar]
- Frick, D.A.; Schuessler, J.A.; Sommer, M.; Blanckenburg, F. Laser Ablation In Situ Silicon Stable Isotope Analysis of Phytoliths. Geostand. Geoanalytical Res. 2019, 43, 77–91. [Google Scholar] [CrossRef]
- Fleck, A.T.; Schulze, S.; Hinrichs, M.; Specht, A.; Waßmann, F.; Schreiber, L.; Schenk, M.K. Silicon Promotes Exodermal Casparian Band Formation in Si-Accumulating and Si-Excluding Species by Forming Phenol Complexes. PLoS ONE 2015, 10, e0138555. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Taherymoosavi, S.; Cheong, S.; Yin, Y.; Akter, R.; Marjo, C.E.; Rich, A.M.; Mitchell, D.R.G.; Fan, X.; Chew, J.; et al. Advanced characterization of biomineralization at plaque layer and inside rice roots amended with iron- and silica-enhanced biochar. Sci. Rep. 2021, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Kumar, R.; Pathak, A.K.; Chauhan, D.K.; Rai, A.K. Laser-Induced Breakdown Spectroscopy and Phytolith Analysis: An Approach to Study the Deposition and Distribution Pattern of Silicon in Different Parts of Wheat (Triticum aestivum L.) Plant. Agric. Res. 2012, 1, 352–361. [Google Scholar] [CrossRef]
- Soukup, M.; Martinka, M.; Bosnić, D.; Čaplovičová, M.; Elbaum, R.; Lux, A. Formation of silica aggregates in sorghum root endodermis is predetermined by cell wall architecture and development. Ann. Bot. 2017, 120, 739–753. [Google Scholar] [CrossRef]
- Quigley, K.M.; Althoff, A.G.; Donati, G.L. Inductively coupled plasma optical emission spectrometry as a reference method for silicon estimation by near infrared spectroscopy and potential application to global-scale studies of plant chemistry. Microchem. J. 2016, 129, 231–235. [Google Scholar] [CrossRef]
- Sucharová, J.; Suchara, I. Determination of 36 elements in plant reference materials with different Si contents by inductively coupled plasma mass spectrometry: Comparison of microwave digestions assisted by three types of digestion mixtures. Anal. Chim. Acta 2006, 576, 163–176. [Google Scholar] [CrossRef]
- Le Blond, J.S.; Strekopytov, S.; Unsworth, C.; Williamson, B.J. Testing a new method for quantifying Si in silica-rich biomass using HF in a closed vessel microwave digestion system. Anal. Methods 2011, 3, 1752–1758. [Google Scholar] [CrossRef]
- Ramírez-Olvera, S.M.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Del Ruíz-Posadas, L.M.; Alcántar-González, E.G.; Saucedo-Veloz, C. Silicon Stimulates Plant Growth and Metabolism in Rice Plants under Conventional and Osmotic Stress Conditions. Plants 2021, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.-H.; Zhan, S.-S.; Wang, S.-Z.; Tang, Y.-T.; Chaney, R.L.; Fang, X.-H.; Cai, X.-D.; Qiu, R.-L. Silicon-mediated amelioration of zinc toxicity in rice (Oryza sativa L.) seedlings. Plant Soil 2012, 350, 193–204. [Google Scholar] [CrossRef]
- May, T.W.; Wiedmeyer, R.H. A Table of Polyatomic Interferences in ICP-MS. At. Spectrosc. 1998, 19, 150–155. [Google Scholar]
- Mihaylova, V.; Lyubomirova, V.; Djingova, R. Optimization of sample preparation and ICP-MS analysis for determination of 60 elements for characterization of the plant ionome. Int. J. Environ. Anal. Chem. 2013, 93, 1441–1456. [Google Scholar] [CrossRef]
- Feng, X.; Wu, S.; Wharmby, A.; Wittmeier, A. Microwave digestion of plant and grain standard reference materials in nitric and hydrofluoric acids for multi-elemental determination by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1999, 14, 939–946. [Google Scholar] [CrossRef]
- Aureli, F.; Ciprotti, M.; D’Amato, M.; Da do Nascimento Silva, E.; Nisi, S.; Passeri, D.; Sorbo, A.; Raggi, A.; Rossi, M.; Cubadda, F. Determination of Total Silicon and SiO2 Particles Using an ICP-MS Based Analytical Platform for Toxicokinetic Studies of Synthetic Amorphous Silica. Nanomaterials 2020, 10, 888. [Google Scholar] [CrossRef]
- Lum, T.-S.; Leung, K.S.-Y. Strategies to overcome spectral interference in ICP-MS detection. J. Anal. At. Spectrom. 2016, 31, 1078–1088. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, S.-J. Dynamic reaction cell inductively coupled plasma mass spectrometry for determination of silicon in steel. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 153–157. [Google Scholar] [CrossRef]
- Klemens, P.; Heumann, K.G. Development of an ICP–HRIDMS method for accurate determination of traces of silicon in biological and clinical samples. Fresenius J. Anal. Chem. 2001, 371, 758–763. [Google Scholar] [CrossRef]
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef]
- Liang, Y.; Hua, H.; Zhu, Y.-G.; Zhang, J.; Cheng, C.; Römheld, V. Importance of plant species and external silicon concentration to active silicon uptake and transport. New Phytol. 2006, 172, 63–72. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.S.; Gonçalves, É.C.B.d.A. Chemical speciation: An instrument for evaluation of mineral bioavailability. Cienc. Rural 2015, 45, 1126–1132. [Google Scholar] [CrossRef]
- Schmitt, C.; Aberkane, L.; Sanchez, C. Protein-polysaccharide complexes and coacervates. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Ltd.: Boca Raton, FL, USA, 2009; pp. 420–476. ISBN 9781845695873. [Google Scholar]
- Ebdon, L.; Foulkes, M.; Fredeen, K.; Hanna, C.; Sutton, K. Silicon speciation using reversed phase high performance liquid chromatography-inductively coupled plasma atomic emission spectrometry—Radial versus axial viewing. Spectrochim. Acta Part B At. Spectrosc. 1998, 53, 859–865. [Google Scholar] [CrossRef]
- Carter, J.; Ebdon, L.; Hywel Evans, E. Speciation of silicon and phosphorous using liquid chromatography coupled with sector field high resolution ICP-MS. Microchem. J. 2004, 76, 35–41. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Park, J.-J.; Kim, K.W.; Park, T.-J.; Park, E.W.; Kim, Y. Solid-state NMR spectroscopy of silicon-treated rice with enhanced host resistance against blast. Anal. Sci. 2006, 22, 645–648. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Paasch, S.; Brunner, E.; Bäucker, E.; Dudel, E.G. Silica uptake from nanoparticles and silica condensation state in different tissues of Phragmites australis. Sci. Total Environ. 2013, 442, 6–9. [Google Scholar] [CrossRef]
- Cabrera, Y.; Cabrera, A.; Larsen, F.H.; Felby, C. Solid-state 29Si NMR and FTIR analyses of lignin-silica coprecipitates. Holzforschung 2016, 70, 709–718. [Google Scholar] [CrossRef]
- Atia, A.A.; El-Nahas, A.M.; Marie, A.M.; Mahdy, L.D.A. Adsorption of Oleic Acid on Silica Gel Derived from Rice Ash Hulls: Experimental and Theoretical Studies. Adsorpt. Sci. Technol. 2006, 24, 797–814. [Google Scholar] [CrossRef]
- Kinrade, S.D.; Del Nin, J.W.; Schach, A.S.; Sloan, T.A.; Wilson, K.L.; Knight, C.T.G. Stable Five- and Six-Coordinated Silicate Anions in Aqueous Solution. Science 1999, 285, 1542–1545. [Google Scholar] [CrossRef] [PubMed]
- Stamm, F.M.; Méheut, M.; Zambardi, T.; Chmeleff, J.; Schott, J.; Oelkers, E.H. Extreme silicon isotope fractionation due to Si organic complexation: Implications for silica biomineralization. Earth Planet. Sci. Lett. 2020, 541, 116287. [Google Scholar] [CrossRef]
- Pu, J.; Wang, L.; Zhang, W.; Ma, J.; Zhang, X.; Putnis, C.V. Organically-bound silicon enhances resistance to enzymatic degradation and nanomechanical properties of rice plant cell walls. Carbohydr. Polym. 2021, 266, 118057. [Google Scholar] [CrossRef]
- Korndörfer, G.H.; Snyder, G.H.; Ulloa, M.; Powell, G.; Datnoff, L.E. Calibration of soil and plant silicon analysis for rice production. J. Plant Nutr. 2001, 24, 1071–1084. [Google Scholar] [CrossRef]
- Inanaga, S.; Okasaka, A.; Tanaka, S. Does silicon exist in association with organic compounds in rice plant? Soil Sci. Plant Nutr. 1995, 41, 111–117. [Google Scholar] [CrossRef]
- Matychenkov, I.V.; Khomyakov, D.M.; Pakhnenko, E.P.; Bocharnikova, E.A.; Matychenkov, V.V. Mobile Si-rich compounds in the soil–plant system and methods for their determination. Mosc. Univ. Soil Sci. Bull. 2016, 71, 120–128. [Google Scholar] [CrossRef]
- Currie, H.A.; Perry, C.C. Silica in Plants: Biological, Biochemical and Chemical Studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.-F.; Legay, S. Silicon and the Plant Extracellular Matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef]
- Ishii, T.; Matsunaga, T. Aqueous Macromolecules with Silicon from Alcoholinsoluble Residues of Rice Seedlings. Jpn. Agric. Res. Q. 2008, 42, 181–186. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Abdullah, S.N.A.; Rafii, M.Y.; Azizi, P.; Nejat, N.; Idris, A.S. Isolation and Expression Analysis of Novel Silicon Absorption Gene from Roots of Mangrove (Rhizophora apiculata) via Suppression Subtractive Hybridization. BioMed Res. Int. 2014, 2014, 971985. [Google Scholar] [CrossRef]
- Kauss, H.; Seehaus, K.; Franke, R.; Gilbert, S.; Dietrich, R.A.; Kröger, N. Silica deposition by a strongly cationic proline-rich protein from systemically resistant cucumber plants. Plant J. 2003, 33, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Del Amo, Y.; Brzezinski, M.A.; Stucky, G.D.; Morse, D.E. A novel fluorescent silica tracer for biological silicification studies. Chem. Biol. 2001, 8, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Dabney, C.; Ostergaard, J.; Watkins, E.; Chen, C. A novel method to characterize silica bodies in grasses. Plant Methods 2016, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Blecher, I.C.; Seidel, R.; Thomann, R.; Speck, T. Comparison of Different Methods for the Detection of Silica Inclusions in Plant Tissues. Int. J. Plant Sci. 2012, 173, 229–238. [Google Scholar] [CrossRef]
- De Cassia Félix Alvarez, R.; de Mello Prado, R.; Felisberto, G.; Deus, A.C.F.; Oliveira, R. Effects of Soluble Silicate and Nanosilica Application on Rice Nutrition in an Oxisol. Pedosphere 2018, 28, 597–606. [Google Scholar] [CrossRef]
- Głazowska, S.; Baldwin, L.; Mravec, J.; Bukh, C.; Hansen, T.H.; Jensen, M.M.; Fangel, J.U.; Willats, W.G.T.; Glasius, M.; Felby, C.; et al. The impact of silicon on cell wall composition and enzymatic saccharification of Brachypodium distachyon. Biotechnol. Biofuels. 2018, 11, 171. [Google Scholar] [CrossRef]
- Hodson, M.J.; Sangster, A.G. The Interaction Between Silicon and Aluminium in Sorghum bicolor (L.) Moench: Growth Analysis and X-ray Microanalysis. Ann. Bot. 1993, 72, 389–400. [Google Scholar] [CrossRef]
- Zexer, N.; Elbaum, R. Unique lignin modifications pattern the nucleation of silica in sorghum endodermis. J. Exp. Bot. 2020, 71, 6818–6829. [Google Scholar] [CrossRef]
- Asgari, F.; Majd, A.; Jonoubi, P.; Najafi, F. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.). Plant Physiol. Biochem. 2018, 127, 152–160. [Google Scholar] [CrossRef]
- Perry, C.C.; Williams, R.J.; Fry, S.C. Cell Wall Biosynthesis during Silicification of Grass Hairs. J. Plant Physiol. 1987, 126, 437–448. [Google Scholar] [CrossRef]
- Perry, C.C.; Fraser, M.A. Silica Deposition and Ultrastructure in the Cell Wall of Equisetum arvense: The Importance of Cell Wall Structures and Flow Control in Biosilicification? Philos. Trans. R. Soc. Lond. B Biol. Sci. 1991, 334, 149–157. [Google Scholar]
- Carrasco-Gil, S.; Rodríguez-Menéndez, S.; Fernández, B.; Pereiro, R.; de La Fuente, V.; Hernandez-Apaolaza, L. Silicon induced Fe deficiency affects Fe, Mn, Cu and Zn distribution in rice (Oryza sativa L.) growth in calcareous conditions. Plant Physiol. Biochem. 2018, 125, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Barisone, G.A.; O’Donnell, R.T.; Ma, Y.; Abuhay, M.W.; Lundeberg, K.; Gowda, S.; Tuscano, J.M. A purified, fermented, extract of Triticum aestivum has lymphomacidal activity mediated via natural killer cell activation. PLoS ONE 2018, 13, e0190860. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Greger, M. A Review on Si Uptake and Transport System. Plants 2019, 8, 81. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Park, B.S.; Adnan, M.; Germ, M.; Kreft, I.; Woo, S.H.; Park, C.H. Silicon biostimulant enhances the growth characteristics and fortifies the bioactive compounds in common and Tartary buckwheat plant. J. Crop Sci. Biotechnol. 2021, 24, 51–59. [Google Scholar] [CrossRef]
- Kaynar, O.; Berktas, Y. How to Choose the Right Plate for Thin-Layer Chromatography? Rev. Anal. Chem. 2010, 29, 129–148. [Google Scholar] [CrossRef]
- Urbain, A.; Simões-Pires, C.A. Thin-layer Chromatography of Plants, with Chemical and Biological Detection Methods. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 1–22. ISBN 9780470027318. [Google Scholar]
- Mathias, E.; Halkar, U. Separation and characterization of lignin compounds from the walnut (Juglans regia) shell oil using preparative TLC, GC–MS and 1H NMR. J. Anal. Appl. Pyrolysis 2004, 71, 515–524. [Google Scholar] [CrossRef]
- Grzelak, E.M.; Hwang, C.; Cai, G.; Nam, J.-W.; Choules, M.P.; Gao, W.; Lankin, D.C.; McAlpine, J.B.; Mulugeta, S.G.; Napolitano, J.G.; et al. Bioautography with TLC-MS/NMR for Rapid Discovery of Anti-tuberculosis Lead Compounds from Natural Sources. ACS Infect. Dis. 2016, 2, 294–301. [Google Scholar] [CrossRef]
- Kisomi, A.S.; Alizadeh, T.; Shakeri, A.; Nouri, A.; Farsadrooh, M.; Najafi AsliPashaki, S. Application of μ-TLC for speciation of inorganic arsenic by laser ablation inductively coupled plasma mass spectrometry. Microchem. J. 2020, 159, 105443. [Google Scholar] [CrossRef]
- Vorapalawut, N.; Martinez Labrador, M.; Pohl, P.; Caetano, M.; Chirinos, J.; Arnaudguilhem, C.; Bouyssiere, B.; Shiowatana, J.; Lobinski, R. Application of TLC and LA ICP SF MS for speciation of S, Ni and V in petroleum samples. Talanta 2012, 97, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Rezić, I.; Špehar, M.; Jakovljević, S. Characterization of Ag and Au nanolayers on Cu alloys by TLC, SEM-EDS, and ICP-OES. Mater. Corros. 2017, 68, 560–565. [Google Scholar] [CrossRef]
- Rodrigues, F.Á.; McNally, D.J.; Datnoff, L.E.; Jones, J.B.; Labbé, C.; Benhamou, N.; Menzies, J.G.; Bélanger, R.R. Silicon Enhances the Accumulation of Diterpenoid Phytoalexins in Rice: A Potential Mechanism for Blast Resistance. Phytopathology 2004, 94, 177–183. [Google Scholar] [CrossRef]
- Sytar, O.; Bośko, P.; Živčák, M.; Brestic, M.; Smetanska, I. Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes. Molecules 2018, 23, 2282. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, J.M.; Jocelyn Paré, J.R.; Sigouin, M. Chapter 2 High performance liquid chromatography (HPLC): Principles and applications. In Instrumental Methods in Food Analysis; Elsevier: Amsterdam, The Netherlands, 1997; pp. 37–59. ISBN 9780444818683. [Google Scholar]
- Rahman, M. Application of computational methods in isolation of plant secondary metabolites. In Computational Phytochemistry; Sarker, S.D., Nahar, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128123645. [Google Scholar]
- Ponce de León, C.A.; Montes-Bayón, M.; Caruso, J.A. Elemental speciation by chromatographic separation with inductively coupled plasma mass spectrometry detection. J. Chromatogr. A 2002, 974, 1–21. [Google Scholar] [CrossRef]
- Lech, K.; Jarosz, M. Novel methodology for the extraction and identification of natural dyestuffs in historical textiles by HPLC–UV–Vis–ESI MS. Case study: Chasubles from the Wawel Cathedral collection. Anal. Bioanal. Chem. 2011, 399, 3241–3251. [Google Scholar] [CrossRef]
- Chawla, G.; Ranjan, C. Principle, Instrumentation, and Applications of UPLC: A Novel Technique of Liquid Chromatography. Open Chem. J. 2016, 3, 1–16. [Google Scholar] [CrossRef]
- Batool, A.; Menaa, F. Concentration and purification of seaweed components by chromatography methods. In Sustainable Seaweed Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 315–370. ISBN 9780128179437. [Google Scholar]
- Rainville, P.D.; Theodoridis, G.; Plumb, R.S.; Wilson, I.D. Advances in liquid chromatography coupled to mass spectrometry for metabolic phenotyping. Trends Anal. Chem. 2014, 61, 181–191. [Google Scholar] [CrossRef]
- Taleuzzaman, M.; Ali, S.; Gilani, S.; Imam, S.; Hafeez, A. Ultra Performance Liquid Chromatography (UPLC)—A Review. Austin J. Anal. Pharm. Chem. 2015, 2, 1056. [Google Scholar]
- Vega, I.; Rumpel, C.; Ruíz, A.; La Mora, M.d.L.; Calderini, D.F.; Cartes, P. Silicon Modulates the Production and Composition of Phenols in Barley under Aluminum Stress. Agronomy 2020, 10, 1138. [Google Scholar] [CrossRef]
- Williams, R.J.P. Introduction to Silicon Chemistry and Biochemistry. In Ciba Foundation Symposium 121—Silicon Biochemistry; Evered, D., O’Connor, M., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 24–39. ISBN 9780470513323. [Google Scholar]
- Fleck, A.T.; Nye, T.; Repenning, C.; Stahl, F.; Zahn, M.; Schenk, M.K. Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J. Exp. Bot. 2011, 62, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, J.; Labbé, C.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Mol. Plant Pathol. 2015, 16, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Karpagasundari, C.; Kulothungan, S. Analysis of bioactive compounds in Physalis minima leaves using GC MS, HPLC, UV-VIS and FTIR techniques. J. Pharmacogn. Phytochem. 2014, 3, 196–201. [Google Scholar]
- Chavan, S.S.; Jadhav, R.S.; Khemanar, K.S.; Tambe, V.B. Evaluation of Antibacterial Activity and Phytochemical Screening of Medicago sativa Leaves. Int. J. Curr. Res. Acad. Rev. 2015, 3, 308–313. [Google Scholar]
- Saniewska, A.; Jurzysta, M.; Biały, Z. Differential antifungal activity of alfalfa (Medicago santva L.) saponins originated from roots and aerial parts for some ornamental plant pathogens. Acta Agrobot. 2001, 54, 31–43. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Argentieri, M.P.; Żuchowski, J.; Biazzi, E.; Tava, A.; Oleszek, W.; Avato, P. Activity of Saponins from Medicago Species against Phytoparasitic Nematodes. Plants 2020, 9, 443. [Google Scholar] [CrossRef]
- Saeed, S.; Tariq, P. Antibacterial activities of Mentha piperita, Pisum sativum and Momordica charantia. Pak. J. Bot. 2005, 37, 997–1001. [Google Scholar]
- Wang, H.X.; Ng, T.B. An antifungal protein from the pea Pisum sativum var. arvense Poir. Peptides 2006, 27, 1732–1737. [Google Scholar] [CrossRef]
- Ye, X.; Ng, T. Isolation of pisumin, a novel antifungal protein from legumes of the sugar snap pea Pisum sativum var. macrocarpon. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Pharmacol. 2003, 134, 235–240. [Google Scholar] [CrossRef]
- Rehman, S.; Khanum, A. Isolation and Characterization of Peptide (S) from Pisum sativum Having Antimicrobial Activity against Various Bacteria. Pak. J. Bot. 2011, 49, 2971–2978. [Google Scholar]
- Erecevit, P.; Kırbağ, S. Determining the Phytochemical Parameters of Pisum sativum (pease) and Effects on the Development of Debaryomyces hansenii. Indian J. Pharm. Educ. Res. 2017, 51, 631–636. [Google Scholar] [CrossRef]
- De Caleya, R.F.; Gonzalez-Pascual, B.; García-Olmedo, F.; Carbonero, P. Susceptibility of Phytopathogenic Bacteria to Wheat Purothionins In Vitro. Appl. Microbiol. 1972, 23, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Caporale, C.; Chilosi, G.; Vacca, F.; Bertini, L.; Magro, P.; Poerio, E.; Buonocore, V. Structural and antifungal properties of a pathogenesis-related protein from wheat kernel. J. Protein Chem. 1996, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; van Parijs, J.; Allen, A.K.; Peumans, W.J. Comparison of some molecular, enzymatic and antifungal properties of chitinases from thorn-apple, tobacco and wheat. Physiol. Mol. Plant Pathol. 1988, 33, 319–331. [Google Scholar] [CrossRef]
- Saha, S.; Islam, Z.; Islam, S.; Hossain, M.; Islam, S.M. Evaluation of antimicrobial activity of wheat (Triticum aestivum L.) against four bacterial strains. SKUAST J. Res. 2018, 20, 58–62. [Google Scholar]
- Rajpurohit, L.; Mehta, N.; Ankola, A.; Gadiyar, A. Evaluation of the anti-microbial activity of various concentration of wheat grass (Triticum aestivum) extract against Gram-positive bacteria: An in vitro study. J. Dent. Res. Rev. 2015, 2, 70–72. [Google Scholar] [CrossRef]
- Schalchli, H.; Pardo, F.; Hormazábal, E.; Palma, R.; Guerrero, J.; Bensch, E. Antifungal activity of wheat root exudate extracts on Gaeumannomyces graminis var. Tritici growth. J. Soil Sci. Plant Nutr. 2012, 12, 329–337. [Google Scholar] [CrossRef]
- Sharma, N.; Tiwari, V.; Vats, S.; Kumari, A.; Chunduri, V.; Kaur, S.; Kapoor, P.; Garg, M. Evaluation of Anthocyanin Content, Antioxidant Potential and Antimicrobial Activity of Black, Purple and Blue Colored Wheat Flour and Wheat-Grass Juice against Common Human Pathogens. Molecules 2020, 25, 5785. [Google Scholar] [CrossRef]
- Rajoria, A.; Mehta, A.; Mehta, P.; Ahirwal, L.; Shukla, S. Phytochemical analysis and estimation of major bioactive compounds from Triticum aestivum L. grass with antimicrobial potential. Pak. J. Pharm. Sci. 2015, 28, 2221–2225. [Google Scholar]
- Choma, I.; Jesionek, W. TLC-Direct Bioautography as a High Throughput Method for Detection of Antimicrobials in Plants. Chromatography 2015, 2, 225–238. [Google Scholar] [CrossRef]
- Borisov, R.; Kanateva, A.; Zhilyaev, D. Recent Advances in Combinations of TLC With MALDI and Other Desorption/Ionization Mass-Spectrometry Techniques. Front. Chem. 2021, 9, 771801. [Google Scholar] [CrossRef] [PubMed]
- Kroslakova, I.; Pedrussio, S.; Wolfram, E. Direct Coupling of HPTLC with MALDI-TOF MS for Qualitative Detection of Flavonoids on Phytochemical Fingerprints. Phytochem. Anal. 2016, 27, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cebolla, V.L.; Jarne, C.; Vela, J.; Garriga, R.; Membrado, L.; Galbán, J. Scanning densitometry and mass spectrometry for HPTLC analysis of lipids: The last 10 years. J. Liq. Chromatogr. Relat. Technol. 2021, 44, 148–170. [Google Scholar] [CrossRef]
- Bañuelos-Hernández, A.E.; Azadniya, E.; Ramírez Moreno, E.; Morlock, G.E. Bioprofiling of Mexican Plectranthus amboinicus (Lour.) essential oil via planar chromatography–effect-directed analysis combined with direct analysis in real time high-resolution mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 344–350. [Google Scholar] [CrossRef]
- Abouzeid, S.; Beutling, U.; Elekhnawy, E.; Selmar, D. Antibacterial and Antibiofilm Effects of Allelopathic Compounds Identified in Medicago sativa L. Seedling Exudate against Escherichia coli. Molecules 2023, 28, 2645. [Google Scholar] [CrossRef]
- Chamachar, M.M.; Fazeli, M.R.; Salimi, M.; Samadi, N. Growth promoting activity, anti-biofilm effect, and down regulation of papC and rcsA genes expression by Medicago sativa (alfalfa) extract. Food Biosci. 2022, 50, 102182. [Google Scholar] [CrossRef]
- Silva, N.B.S.; Marques, L.A.; Röder, D.D.B. Antibiofilm Activity of Natural Products: Promising Strategies for Combating Microbial Biofilms. Ann. Trop. Med. Public Health 2020, 4, 92–99. [Google Scholar] [CrossRef]
- Zainab, M.H.; Zainab, S.H.; Sraa, N.M.; Elaf, S.M.; Hala, M.S. Antibiofilm formation activity of purified lectin from wheat seeds (Triticum aestivum) against Candida albicans causing oral candidiasis. Tex. J. Multidiscip. Stud. 2022, 15, 150–156. [Google Scholar]
- González-Ortiz, G.; van Quarles Ufford, H.C.; Halkes, S.B.A.; Cerdà-Cuéllar, M.; Beukelman, C.J.; Pieters, R.J.; Liskamp, R.M.J.; Pérez, J.F.; Martín-Orue, S.M. New properties of wheat bran: Anti-biofilm activity and interference with bacteria quorum-sensing systems. Environ. Microbiol. 2014, 16, 1346–1353. [Google Scholar] [CrossRef]
- Gatouillat, G.; Alabdul Magid, A.; Bertin, E.; Okiemy-Akeli, M.-G.; Morjani, H.; Lavaud, C.; Madoulet, C. Cytotoxicity and Apoptosis Induced by Alfalfa (Medicago sativa) Leaf Extracts in Sensitive and Multidrug-Resistant Tumor Cells. Nutr. Cancer 2014, 66, 483–491. [Google Scholar] [CrossRef]
- Gatouillat, G.; Magid, A.A.; Bertin, E.; El btaouri, H.; Morjani, H.; Lavaud, C.; Madoulet, C. Medicarpin and millepurpan, two flavonoids isolated from Medicago sativa, induce apoptosis and overcome multidrug resistance in leukemia P388 cells. Phytomedicine 2015, 22, 1186–1194. [Google Scholar] [CrossRef]
- Avato, P.; Migoni, D.; Argentieri, M.; Fanizzi, F.P.; Tava, A. Activity of Saponins from Medicago species Against HeLa and MCF-7 Cell Lines and their Capacity to Potentiate Cisplatin Effect. Anticancer Agents Med. Chem. 2017, 17, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Lanza, A.; Tava, A.; Catalano, M.; Ragona, L.; Singuaroli, I.; Della Robustelli Cuna, F.S.; Della Robustelli Cuna, G. Effects of the Medicago scutellata trypsin inhibitor (MsTI) on cisplatin-induced cytotoxicity in human breast and cervical cancer cells. Anticancer Res. 2004, 24, 227–233. [Google Scholar] [PubMed]
- El-Feky, A.M.; Elbatanony, M.M.; Mounier, M.M. Anti-cancer potential of the lipoidal and flavonoidal compounds from Pisum sativum and Vicia faba peels. Egypt. J. Basic Appl. Sci. 2018, 5, 258–264. [Google Scholar] [CrossRef]
- Stanisavljević, N.S.; Ilić, M.D.; Matić, I.Z.; Jovanović, Ž.S.; Čupić, T.; Dabić, D.Č.; Natić, M.M.; Tešić, Ž.L. Identification of Phenolic Compounds from Seed Coats of Differently Colored European Varieties of Pea (Pisum sativum L.) and Characterization of Their Antioxidant and In Vitro Anticancer Activities. Nutr. Cancer 2016, 68, 988–1000. [Google Scholar] [CrossRef]
- Abd El-Galil, A.A.; Negm El-Dein, A.; Awad, H.M.; Helmy, W.A. Chemical composition and biological activities of aqueous extracts and their sulfated derivatives of pea peel (Pisum sativum L.). Biocatal. Agric. Biotechnol. 2021, 35, 102077. [Google Scholar] [CrossRef]
- Khalaf, Z.A. Extraction and Purification of Asparaginase Enzyme from Pisum sativum Plant and Studying Their Cytotoxicity against L20B Tumor Cell Line. Master’s Thesis, Al-Nahrain University, Baghdad, Iraq, 2012. [Google Scholar]
- El-Aassar, M.R.; Hafez, E.E.; El-Deeb, N.M.; Fouda, M.M. Microencapsulation of lectin anti-cancer agent and controlled release by alginate beads, biosafety approach. Int. J. Biol. Macromol. 2014, 69, 88–94. [Google Scholar] [CrossRef]
- Patel, A. Isolation, characterization and production of a new recombinant lectin protein from leguminous plants. Bio. Chem. Comp. 2014, 2, 2. [Google Scholar] [CrossRef]
- Clemente, A.; Gee, J.M.; Johnson, I.T.; MacKenzie, D.A.; Domoney, C. Pea (Pisum sativum L.) Protease Inhibitors from the Bowman−Birk Class Influence the Growth of Human Colorectal Adenocarcinoma HT29 Cells in Vitro. J. Agric. Food Chem. 2005, 53, 8979–8986. [Google Scholar] [CrossRef]
- Vincentini, O.; Maialetti, F.; Gazza, L.; Silano, M.; Dessi, M.; de Vincenzi, M.; Pogna, N.E. Environmental factors of celiac disease: Cytotoxicity of hulled wheat species Triticum monococcum, T. turgidum ssp. dicoccum and T. aestivum ssp. spelta. J. Gastroenterol. Hepatol. 2007, 22, 1816–1822. [Google Scholar] [CrossRef]
- Rajoria, A.; Mehta, A.; Mehta, P.; Ahirwal, L.; Shukla, S.; Bajpai, V.K. Evaluation of antiproliferative and hepatoprotective effects of wheat grass (Triticum aestivum). Acta Biol. Hung. 2017, 68, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.B. Anticancer & Cytotoxic potential of aqueous extract of Triticum aestivum on HeLa cell line. J. Drug Deliv. Ther. 2016, 6, 84–89. [Google Scholar] [CrossRef]
- Patel, J.B.; Patel, P.; Parmar, R.S.; Patel, D. Anticancer and Cytotoxic Potential of Aqueous Extract of Triticum aestivum on Colorectal Carcinoma. J. Drug Deliv. Ther. 2019, 9, 164–169. [Google Scholar]
- Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T. Antioxidant Activity and Cytotoxicity of Medicago sativa L. Seeds and Herb Extract on Skin Cells. BioResearch Open Access 2020, 9, 229–242. [Google Scholar] [CrossRef]
- Clemente, A.; Carmen Marín-Manzano, M.; Jiménez, E.; Carmen Arques, M.; Domoney, C. The anti-proliferative effect of TI1B, a major Bowman–Birk isoinhibitor from pea (Pisum sativum L.), on HT29 colon cancer cells is mediated through protease inhibition. Br. J. Nutr. 2012, 108, S135–S144. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, M.T.; Mendes, C.B.D.M.; Fischer, G.; Varela Júnior, A.S.; Fernandes, C.G.; Nobre, M.D.O. Triticum aestivum in open skin wounds: Cytotoxicity and collagen histopathology. SCA 2018, 39, 1547. [Google Scholar] [CrossRef]






| Stress | Example | Plant Species | Role of Si |
|---|---|---|---|
| Abiotic stress | |||
| Drought | - | T. aestivum L. | Improves the water relations of wheat leaves, photosynthetic gas exchange, and carboxylase activities in field drought conditions. |
| Salinity | - | T. aestivum L. | Improves the defense by antioxidant enzymes |
| Salinity | - | M. sativa L. | Regulates the K+/Na+ homeostasis and the leaf water balance, also improving antioxidant enzymes’ performance in leaves. |
| Heavy metals | Cd | P. sativum L. | Accumulation of glyoxalase; it enhances the uptake of macronutrients and micronutrients in shoots and roots |
| M. sativa L. | Cd binding in both roots and shoots | ||
| Biotic stress | |||
| Fungal, bacterial, virus, and herbivores diseases | Powdery mildew (Blumeria graminis) | T. aestivum L. | Causes a higher concentration of elicitors |
| Plant Species | Sample Preparation | Techniques | Reference |
|---|---|---|---|
| apple, corn, peach, pepper, watermelon, and bluegrass ground leaves | 200 mg of plant tissue with 10 mL of 1 M HCl + 20 mL of 2.3 M HF for 15 h | ICP analysis | [87] |
| cherry laurel, potato, alfalfa, carnation sunflower, barley grain, grass, French bean, bokashi, oil palm leaf | Plant material at pyrolysis at 420 °C was used | ETV-ICP-OES | [88] |
| rice (O. sativa L.) | Plant tissue added to 2 mL of 30% H2O2 and 4 mL of 50% NaOH in the oven (95 °C) for 4 h. After, was added 1 mL of 5 mM ammonium fluoride (NH4F) | OID MBC using a UV visible spectrophotometer | [89,90,91] |
| wheat (T. aestivum L.) | [90,91] | ||
| plant material (29 samples) | 30 mg of pulverized plant material was incubated for 4 h in 0.1 M Na2CO3 at 80 °C. Afterward, 10 mL of the extract was filtered | NIRS | [86] |
| sugar maple (Acer saccharum Marsh.), American beech (Fagus grandifolia Erhr.) yellow birch (Betula alleghaniensis Britt.) | 100 mg of dried tissue was digested in 40 mL of 0.5 M NaOH at 85 °C for 4 h. 30 mg of dry tissue was digested in 40 mL of 0.1 M Na2CO3 at 85 °C for 4 h | DSi colorimetrically, using the molybdate-blue method | [84,92] |
| C. epigejos and P. australis | First digestion: 100 mg plant material digested in a mixture of 4 mL distilled water, 5 mL nitric acid (65%) and 1 mL hydrofluoric acid (40%) at 190 °C. Second digestion: to neutralize the hydrofluoric acid, was used 10 mL of a 4% boric acid solution at 150 °C | ICP-OES | [81,92] |
| Dillenia suffruticosa, Dipterocarpus globosus, Macaranga trachyphylla, Shorea ochracea | Plant material ashed at 450 °C. The ash is mixed with lithium meta-tetraborate at 1000 °C. The resulting bead was transferred into 10% nitric acid. | ICP-OES | [80,92] |
| D. caespitosa, Lolium perenne, T. aestivum | 100 mg plant material homogenized to a powder; calibration was required. | XRF | [77,92] |
| Plant | Extraction Technique | Solvent | Conditions | Compounds | Reference |
|---|---|---|---|---|---|
| Medicago sativa L. | SFE | supercritical carbon dioxide | 10 mL of 96% ethanol solution for 3 h at 40, 60, and 80 °C in the dark, and it was incubated in water bath with stirring | phenolics and flavonoids | [10] |
| Medicago sativa L. | Maceration | ethanol | 20% aqueous ethanol (v/v) overnight. After, anhydrous ethanol was added into the soak solution to obtain 75% ethanol solution, pretreated at 50 °C with stirring for 1 h. The extracting solution was filtered, and the filter residue was extracted twice more by following the same steps as above. | flavonoids | [108] |
| T. aestivum L. | HRE | glycerin:water | 0.5 g of grounded spelt was subjected to extraction with 5 mL solvent under heat reflux extraction at 60 °C in a water bath for 4 h | phenolics | [7] |
| MAE | 0.5 g of grounded spelt was subjected to extraction with 5 mL solvent under microwave-assisted extraction for 1 min in a microwave oven (LG MS-197H) at output power 700 W for 30 s | ||||
| UAE | 0.5 g of grounded spelt was subjected to extraction with 5 mL solvent under ultrasound-assisted extraction for 30 min in an ultrasonic bath with frequency 50/60 Hz and power 310 W | ||||
| P. sativum L. | Maceration | acetone-water | 700 mL/L aqueous acetone for 30 min attraction of (1:7 w/v) in a shaking incubator. | phenolics | [109] |
| P. sativum L. (pea flour) | SFE | supercritical carbon dioxide + ethanol extraction | 22% ethanol, 86 °C, and 42.71 MPa, 40-min total extraction, including a 10-min static and a 30-min dynamic extraction at a flow rate of 2 mL/min | volatile compounds (1-hexanol, 1-octanol, 1-nonanol, nonanal, and 2-alkyl methoxypyrazines) | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzoł, A.; Cruzado-Tafur, E.; Gołębiowski, A.; Rogowska, A.; Pomastowski, P.; Górecki, R.J.; Buszewski, B.; Szultka-Młyńska, M.; Głowacka, K. Comprehensive Study of Si-Based Compounds in Selected Plants (Pisum sativum L., Medicago sativa L., Triticum aestivum L.). Molecules 2023, 28, 4311. https://doi.org/10.3390/molecules28114311
Orzoł A, Cruzado-Tafur E, Gołębiowski A, Rogowska A, Pomastowski P, Górecki RJ, Buszewski B, Szultka-Młyńska M, Głowacka K. Comprehensive Study of Si-Based Compounds in Selected Plants (Pisum sativum L., Medicago sativa L., Triticum aestivum L.). Molecules. 2023; 28(11):4311. https://doi.org/10.3390/molecules28114311
Chicago/Turabian StyleOrzoł, Aleksandra, Edith Cruzado-Tafur, Adrian Gołębiowski, Agnieszka Rogowska, Paweł Pomastowski, Ryszard J. Górecki, Bogusław Buszewski, Małgorzata Szultka-Młyńska, and Katarzyna Głowacka. 2023. "Comprehensive Study of Si-Based Compounds in Selected Plants (Pisum sativum L., Medicago sativa L., Triticum aestivum L.)" Molecules 28, no. 11: 4311. https://doi.org/10.3390/molecules28114311
APA StyleOrzoł, A., Cruzado-Tafur, E., Gołębiowski, A., Rogowska, A., Pomastowski, P., Górecki, R. J., Buszewski, B., Szultka-Młyńska, M., & Głowacka, K. (2023). Comprehensive Study of Si-Based Compounds in Selected Plants (Pisum sativum L., Medicago sativa L., Triticum aestivum L.). Molecules, 28(11), 4311. https://doi.org/10.3390/molecules28114311