Development and Evaluation of an FDM Printed Nasal Device for CPZ Solid Nanoparticles
Abstract
:1. Introduction
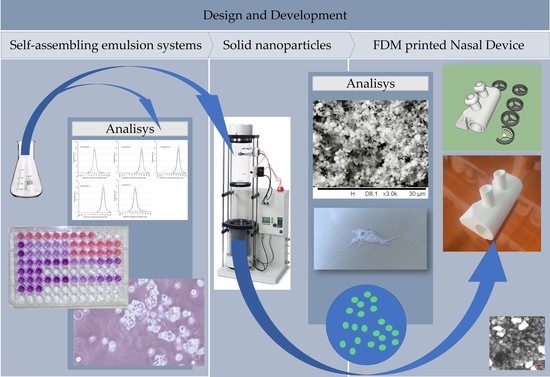
2. Results
2.1. Formulation of Self-Assembling Emulsion Systems
2.2. Setting the Formulation Parameters
2.3. Evaluation of the Size and Size Distribution of Dispersed Droplets
2.4. Evaluation of the Size and Morphology of Solid Nanostructures
2.5. Investigation of the Distribution and Aggregation Properties of Solid Nanoparticles in Primary Containers
2.6. Investigation of the Cytotoxic Effect of Components and Their Blends on RPMI 2650 Nasal Epithelial Cells Using an MTT Viability Assay
2.7. Dissolution of CPZ from the Formulated Nanocarriers
2.8. Development and Pilot Production of a Medical Device for Nasal Delivery Systems
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Formulation of SNEDD Systems
4.3. Formulation of Solid Nano Carriers
4.4. Scanning Electron Microscopy
4.5. Determination of Droplet Size
4.6. Fused Deposition Modeling
4.7. CPZ Content Determination
4.8. Cell Culturing
4.9. MTT Viability Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hanson, L.R.; Frey, W.H. Intranasal Delivery Bypasses the Blood-Brain Barrier to Target Therapeutic Agents to the Central Nervous System and Treat Neurodegenerative Disease. BMC Neurosci. 2008, 9, S5. [Google Scholar] [CrossRef]
- Eleni, K.; Paraskevi, P.; Georgia, V. Advances in intranasal vaccine delivery: A promising non-invasive route of immunization. Vaccine, 2023; in press. [Google Scholar] [CrossRef]
- Ozsoy, Y.; Gungor, S.; Cevher, E. Nasal Delivery of High Molecular Weight Drugs. Molecules 2009, 14, 3754–3779. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics 2022, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.Y.Z.; Soh, S.E.; Chng, S.Y.; Shek, L.P.-C.; Goh, D.Y.T.; Van Bever, H.P.S.; Lee, B.W. Compliance with Topical Nasal Medication—An Evaluation in Children with Rhinitis. Pediatr. Allergy Immunol. 2010, 21, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Bonaccorso, A.; Puglisi, G. Epilepsy Disease and Nose-to-Brain Delivery of Polymeric Nanoparticles: An Overview. Pharmaceutics 2019, 11, 118. [Google Scholar] [CrossRef]
- Harrold, M.W.; Chang, Y.A.; Wallace, R.A.; Farooqui, T.; Wallace, L.J.; Uretsky, N.; Miller, D.D. Charged Analogs of Chlorpromazine as Dopamine Antagonists. J. Med. Chem. 1987, 30, 1631–1635. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Cui, Y.; Chen, Y.; Wu, H.; Wang, J.; Gong, X.; Gao, X.; Yang, L.; Li, J.; et al. Novel chlorpromazine derivatives as anti-endometrial carcinoma agents with reduced extrapyramidal side effects. Bioorganic Chem. 2022, 127, 106008. [Google Scholar] [CrossRef]
- Mackay, E.V. Progressive chlorpromazine jaundice during pregnancy. Med. J. Aust. 1960, 1, 209–212. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Hoffmann, C.; Shen, C.; Le Tourneau, C. Nanoparticle Therapy for Head and Neck Cancers. Curr. Opin. Oncol. 2022, 34, 177–184. [Google Scholar] [CrossRef]
- Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; St. Pourçain, C.B.; Garnett, M.C. Novel Functionalized Biodegradable Polymers for Nanoparticle Drug Delivery Systems. Biomacromolecules 2005, 6, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- LESLIE, R. Surface-Active Agents. Manuf. Chem. Aerosol News 1947, 18, 149–150. [Google Scholar]
- Rundle, C.W.; Presley, C.L.; Militello, M.; Barber, C.; Powell, D.L.; Jacob, S.E.; Atwater, A.R.; Watsky, K.L.; Yu, J.; Dunnick, C.A. Hand Hygiene during COVID-19: Recommendations from the American Contact Dermatitis Society. J. Am. Acad. Dermatol. 2020, 83, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Alshweiat, A.; Ambrus, R.; Csóka, I. Intranasal Nanoparticulate Systems as Alternative Route of Drug Delivery. Curr. Med. Chem. 2019, 26, 6459–6492. [Google Scholar] [CrossRef]
- Sibinovska, N.; Žakelj, S.; Trontelj, J.; Kristan, K. Applicability of RPMI 2650 and Calu-3 Cell Models for Evaluation of Nasal Formulations. Pharmaceutics 2022, 14, 369. [Google Scholar] [CrossRef]
- Mazayen, Z.M.; Ghoneim, A.M.; Elbatanony, R.S.; Basalious, E.B.; Bendas, E.R. Pharmaceutical Nanotechnology: From the Bench to the Market. Futur. J. Pharm. Sci. 2022, 8, 12. [Google Scholar] [CrossRef]
- Strojewski, D.; Krupa, A. Spray Drying and Nano Spray Drying as Manufacturing Methods of Drug-Loaded Polymeric Particles. Polym. Med. 2022, 52, 101–111. [Google Scholar] [CrossRef]
- Harsha, S.; E Aldhubiab, B.; Nair, A.; Abdulrahman Alhaider, I.; Attimarad, M.; Narayanaswamay, V.; Srinivasan, V.; Gangadhara, N.; Asif, A. Nanoparticle formulation by Büchi B-90 Nano Spray Dryer for oral mucoadhesion. Drug Des. Devel. Ther. 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Ahmed, H.H.; Shalby, A.B. Selenium Overcomes Doxorubicin Resistance in Their Nano-Platforms Against Breast and Colon Cancers. Biol. Trace Elem. Res. 2020, 193, 377–389. [Google Scholar] [CrossRef]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning Electron Microscopy. Curr. Protoc. Microbiol. 2012, 25, 2B.2.1–2B.2.47. [Google Scholar] [CrossRef]
- Marx, D.; Williams, G.; Birkhoff, M. Intranasal Drug Administration—An Attractive Delivery Route for Some Drugs. In Drug Discovery and Development—From Molecules to Medicine; InTech: Houston, TX, USA, 2015. [Google Scholar]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Posadas, Y.; Miranda, J.; Uc, P.; Ortega-Olvera, J.; Hernández, S. Strategies That Target Tight Junctions for Enhanced Drug Delivery. Curr. Pharm. Des. 2016, 22, 5313–5346. [Google Scholar] [CrossRef]
- Reichl, S.; Becker, K. Cultivation of RPMI 2650 Cells as an In-Vitro Model for Human Transmucosal Nasal Drug Absorption Studies: Optimization of Selected Culture Conditions. J. Pharm. Pharmacol. 2012, 64, 1621–1630. [Google Scholar] [CrossRef]
- SCHLADITZ, K. Quantitative Micro-CT. J. Microsc. 2011, 243, 111–117. [Google Scholar] [CrossRef]
- Moghimipour, E.; Salimi, A.; Karami, M.; Isazadeh, S. Preparation and Characterization of Dexamethasone Microemulsion Based on Pseudoternary Phase Diagram. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 105–112. [Google Scholar] [CrossRef]
- Boc, S.; Momin, M.A.M.; Farkas, D.R.; Longest, W.; Hindle, M. Development and Characterization of Excipient Enhanced Growth (EEG) Surfactant Powder Formulations for Treating Neonatal Respiratory Distress Syndrome. AAPS Pharm. Sci. Tech. 2021, 22, 136. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, C.; Mullen, T.J.; Hohman, J.N.; Anderson, M.E.; Dameron, A.A.; Andrews, A.M.; Dickey, E.C.; Horn, M.W.; Weiss, P.S. Scanning Electron Microscopy of Nanoscale Chemical Patterns. ACS Nano 2007, 1, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, K. Nanosize Particle Analysis by Dynamic Light Scattering (DLS). Yakugaku Zasshi 2019, 139, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Balletti, C.; Ballarin, M.; Guerra, F. 3D Printing: State of the Art and Future Perspectives. J. Cult. Herit. 2017, 26, 172–182. [Google Scholar] [CrossRef]
- Sibinovska, N.; Žakelj, S.; Kristan, K. Suitability of RPMI 2650 Cell Models for Nasal Drug Permeability Prediction. Eur. J. Pharm. Biopharm. 2019, 145, 85–95. [Google Scholar] [CrossRef]
- Pilicheva, B.; Draganova-Filipova, M.; Zagorchev, P.; Kassarova, M. Investigation of Betahistine Dihydrochloride Biocompatibility and Nasal Permeability In Vitro. J. Appl. Biomed. 2016, 14, 299–305. [Google Scholar] [CrossRef]











| Composition | Excipients | Ratio |
|---|---|---|
| Composition 1 | Lauroglycol FCC | 1:1:1 |
| Cremophor EL | ||
| Transcutol HP | ||
| Composition 2 | Miglyol 840 | 1:1:0.3:1 |
| Cremophor EL | ||
| DMSO | ||
| Glycerol | ||
| Composition 3 | Labrafil 1944 | 1:1:1:1 |
| Labrasol | ||
| Cremophor EL | ||
| Propylene Glycol | ||
| Composition 4 | Labrasol | 1:1:1:0.3:1 |
| Transcutol | ||
| Cremophor EL | ||
| DMSO | ||
| Glycerol | ||
| Composition 5 | Lauroglycol 90 | 1:1:1 |
| Cremophor EL | ||
| Transcutol HP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
To Quoc, T.; Bíró, K.; Pető, Á.; Kósa, D.; Sinka, D.; Lekli, I.; Kiss-Szikszai, A.; Budai, I.; Béres, M.; Vecsernyés, M.; et al. Development and Evaluation of an FDM Printed Nasal Device for CPZ Solid Nanoparticles. Molecules 2023, 28, 4406. https://doi.org/10.3390/molecules28114406
To Quoc T, Bíró K, Pető Á, Kósa D, Sinka D, Lekli I, Kiss-Szikszai A, Budai I, Béres M, Vecsernyés M, et al. Development and Evaluation of an FDM Printed Nasal Device for CPZ Solid Nanoparticles. Molecules. 2023; 28(11):4406. https://doi.org/10.3390/molecules28114406
Chicago/Turabian StyleTo Quoc, Thinh, Krisztina Bíró, Ágota Pető, Dóra Kósa, Dávid Sinka, István Lekli, Attila Kiss-Szikszai, István Budai, Mónika Béres, Miklós Vecsernyés, and et al. 2023. "Development and Evaluation of an FDM Printed Nasal Device for CPZ Solid Nanoparticles" Molecules 28, no. 11: 4406. https://doi.org/10.3390/molecules28114406
APA StyleTo Quoc, T., Bíró, K., Pető, Á., Kósa, D., Sinka, D., Lekli, I., Kiss-Szikszai, A., Budai, I., Béres, M., Vecsernyés, M., Fehér, P., Bácskay, I., & Ujhelyi, Z. (2023). Development and Evaluation of an FDM Printed Nasal Device for CPZ Solid Nanoparticles. Molecules, 28(11), 4406. https://doi.org/10.3390/molecules28114406