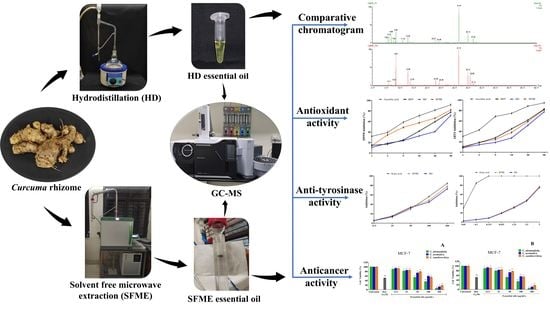
Variation in Yield, Chemical Composition and Biological Activities of Essential Oil of Three Curcuma Species: A Comparative Evaluation of Hydrodistillation and Solvent-Free Microwave Extraction Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Extraction Parameters and Essential Oil Yield
2.2. Comparison of Chemical Composition Obtained by HD and SFME
| Sl No. | RIexp i | RIlit ii | Compound iii | Peak Area % | |||||
|---|---|---|---|---|---|---|---|---|---|
| Curcuma alismatifolia | Curcuma aromatica | Curcuma xanthorrhiza | |||||||
| HD | SFME | HD | SFME | HD | SFME | ||||
| 1 | 926 | 926 | Tricyclene | 0.13 ± 0.02 a | 0.10 ± 0.03 b | 0.13 ± 0.04 a | 0.14 ± 0.05 a | - | - |
| 2 | 930 | 930 | α-Thujene | 1.32 ± 0.14 a | 0.23 ± 0.05 cd | 1.15 ± 0.0 b | 0.12 ± 0.02 de | 0.27 ± 0.06 c | 0.07 ± 0.01 e |
| 3 | 946 | 939 | α-Pinene | 3.18 ± 0.27 a | 1.40 ± 0.18 b | 0.08 ± 0.02 a | 0.10 ± 0.03 e | 1.03 ± 0.14 c | 0.74 ± 0.14 d |
| 4 | 950 | 954 | Camphene | 0.88 ± 0.12 c | 0.91 ± 0.13 c | 3.28 ± 0.31 a | 1.61 ± 0.25 b | 0.35 ± 0.09 e | 0.78 ± 0.10 d |
| 5 | 974 | 975 | Sabinene | 0.31 ± 0.04 c | 0.50 ± 0.11 b | 0.82 ± 0.15 a | 0.10 ± 0.05 d | - | - |
| 6 | 984 | 979 | β-Pinene | 4.96 ± 0.32 a | 2.86 ± 0.24 b | 0.88 ± 0.14 d | 0.08 ± 0.05 f | 0.25 ± 0.09 e | 1.73 ± 0.22 c |
| 7 | 1003 | 990 | Myrcene | 0.07 ± 0.01 a | 0.08 ± 0.02 a | - | - | - | - |
| 8 | 1006 | 1011 | δ-3-Carene | 1.58 ± 0.14 a | 0.09 ± 0.03 b | - | - | - | - |
| 9 | 1020 | 1024 | p-Cymene | 0.15 ± 0.03 a | 0.13 ± 0.02 a | - | - | - | - |
| 10 | 1025 | 1029 | Limonene | 1.00 ± 0.15 b | 1.67 ± 0.18 a | 1.80 ± 0.21 a | 0.40 ± 0.08 c | 0.25 ± 0.07 cd | 0.17 ± 0.02 d |
| 11 | 1029 | 1031 | 1,8-cineole | 6.47 ± 0.78 c | 16.60 ± 1.45 a | 13.02 ± 1.32 b | 7.55 ± 0.88 c | 0.80 ± 0.18 d | 0.81 ± 0.17 d |
| 12 | 1051 | 1059 | γ-Terpinene | - | - | 0.18 ± 0.04 a | 0.04 ± 0.01 b | - | - |
| 13 | 1081 | 1088 | Terpinolene | 0.14 ± 0.03 a | 0.10 ± 0.01 c | 0.27 ± 0.04 b | 0.29 ± 0.07 a | 0.45 ± 0.05 a | 0.17 ± 0.02 c |
| 14 | 1085 | 1090 | 2-Nonanone | 0.19 ± 0.05 a | 0.21 ± 0.05 a | - | - | - | - |
| 15 | 1097 | 1096 | Linalool | 1.93 ± 0.18 b | 0.73 ± 0.09 c | 2.87 ± 0.47 a | 2.99 ± 0.37 a | 0.40 ± 0.09 d | 0.78 ± 0.11 c |
| 16 | 1113 | 1114 | trans-Thujone | 0.10 ± 0.02 a | 0.12 ± 0.04 | - | - | - | - |
| 17 | 1143 | 1146 | Camphor | 3.78 ± 0.74 d | 9.61 ± 1.24 b | 8.45 ± 1.22 c | 14.04 ± 2.01 a | 3.33 ± 0.58 e | 2.71 ± 0.42 f |
| 18 | 1157 | 1160 | Isoborneol | - | - | - | - | 0.14 ± 0.05 a | 0.68 ± 0.15 a |
| 19 | 1159 | 1166 | δ-Terpineol | 1.50 ± 0.24 a | 0.12 ± 0.05 b | - | - | - | - |
| 20 | 1160 | 1168 | 3-Thujanol | 0.29 ± 0.07 a | 4.35 ± 0.53 a | - | - | - | - |
| 21 | 1160 | 1169 | Borneol | - | - | 2.90 ± 0.14 b | 6.04 ± 0.41 a | 0.15 ± 0.02 d | 0.56 ± 0.07 c |
| 22 | 1174 | 1177 | Terpinen-4-ol | 0.57 ± 0.19 b | 0.35 ± 0.14 d | 0.80 ± 0.22 a | 0.43 ± 0.14 c | 0.08 ± 0.01 f | 0.26 ± 0.08 e |
| 23 | 1192 | 1188 | α-Terpineol | 0.19 ± 0.04 d | 0.60 ± 0.17 c | 0.90 ± 0.10 b | 1.02 ± 0.11 a | 0.07 ± 0.01 e | 0.61 ± 0.04 c |
| 24 | 1272 | 1267 | Geranial | 0.43 ± 0.06 b | 0.63 ± 0.10 a | - | - | - | - |
| 25 | 1281 | 1285 | Isobornyl acetate | - | - | 0.07 ± 0.05 a | 0.05 ± 0.01 a | - | - |
| 26 | 1287 | 1285 | Bornyl acetate | 0.12 ± 0.08 a | 0.09 ± 0.02 a | - | - | - | - |
| 27 | 1290 | 1294 | 2-Undecanone | 0.18 ± 0.03 a | 0.10 ± 0.04 a | - | - | - | - |
| 28 | 1328 | 1338 | δ-Elemene | 0.12 ± 0.03 d | 0.18 ± 0.05 c | 0.61 ± 0.09 a | 0.07 ± 0.01 e | 0.18 ± 0.09 a | 0.45 ± 0.08 b |
| 29 | 1371 | 1351 | α-Cubebene | - | - | - | - | 1.88 ± 0.29 a | 1.89 ± 0.34 a |
| 30 | 1375 | 1375 | α-Ylangene | 0.17 ± 0.04 a | 0.24 ± 0.06 b | - | - | - | - |
| 31 | 1391 | 1376 | α-Copaene | - | - | 0.44 ± 0.08 b | 1.13 ± 0.12 a | - | - |
| 32 | 1395 | 1390 | β-Elemene | 1.30 ± 0.14 d | 3.34 ± 0.21 b | 4.03 ± 0.19 a | 1.85 ± 0.14 c | - | - |
| 33 | 1409 | 1408 | (Z)-Caryophyllene | - | - | 0.21 ± 0.09 a | 0.36 ± 0.09 c | 1.84 ± 0.21 a | 0.54 ± 0.10 b |
| 34 | 1411 | 1411 | α-Cedrene | 1.55 ± 0.24 b | 1.97 ± 0.25 a | 0.09 ± 0.03 a | 0.07 ± 0.01 c | - | - |
| 35 | 1426 | 1419 | β-Caryophyllene | 0.26 ± 0.05 c | 0.36 ± 0.08 b | 1.69 ± 0.22 a | 0.26 ± 0.07 a | - | - |
| 36 | 1427 | 1434 | trans-α-Bergamotene | 0.27 ± 0.05 a | 0.23 ± 0.07 a | 0.12 ± 0.04 b | 0.08 ± 0.02 c | ||
| 37 | 1439 | 1436 | γ-Elemene | 0.24 ± 0.05 a | 0.05 ± 0.01 c | - | - | 0.20 ± 0.08 b | 0.18 ± 0.02 b |
| 38 | 1444 | 1439 | α-Guaiene | - | - | 0.45 ± 0.18 a | 0.22 ± 0.10 a | - | - |
| 39 | 1444 | 1441 | Aromadendrene | 0.63 ± 0.21 a | 0.19 ± 0.08 b | - | - | - | - |
| 40 | 1446 | 1442 | (Z)-β-Farnesene | - | - | 0.46 ± 0.04 a | 0.18 ± 0.08 a | 0.23 ± 0.07 b | 0.08 ± 0.04 c |
| 41 | 1463 | 1456 | (E)-β-Farnesene | - | - | - | - | 0.25 ± 0.03 b | 0.87 ± 0.09 a |
| 42 | 1465 | 1460 | allo-Aromadendrene | - | - | - | - | 0.27 ± 0.04 a | 0.22 ± 0.08 |
| 43 | 1470 | 1466 | cis-Muurola-4(14),5-diene | - | - | - | - | 0.63 ± 0.14 a | 0.11 ± 0.04 |
| 44 | 1478 | 1477 | γ-Gurjunene | 0.05 ± 0.01 | 0.64 ± 0.21 b | - | - | 1.63 ± 0.30 a | 0.54 ± 0.14 c |
| 45 | 1479 | 1480 | ar-Curcumene | 0.77 ± 0.12 | 1.07 ± 0.14 d | 1.85 ± 0.24 c | 0.77 ± 0.15 e | 3.27 ± 0.66 a | 2.96 ± 0.46 b |
| 46 | 1484 | 1481 | Germacrene D | - | - | 0.63 ± 0.14 a | 0.04 ± 0.01 b | - | - |
| 47 | 1492 | 1490 | β-Selinene | - | - | 0.25 ± 0.07 b | 0.21 ± 0.04 | 0.18 ± 0.08 c | 0.42 ± 0.12 a |
| 48 | 1492 | 1493 | cis-β-Guaiene | - | - | - | - | 0.13 ± 0.02 b | 0.33 ± 0.11 a |
| 49 | 1496 | 1493 | α-Zingiberene | 1.92 ± 0.36 a | 0.65 ± 0.25 d | 0.89 ± 0.10 b | 0.08 ± 0.04 | 0.71 ± 0.09 c | 0.56 ± 0.08 e |
| 50 | 1503 | 1499 | Curzerene | 0.11 ± 0.04 | 0.19 ± 0.05 d | 4.18 ± 0.74 a | 2.02 ± 0.34 b | 0.21 ± 0.11 | 0.48 ± 0.08 c |
| 51 | 1505 | 1500 | β-Himachalene | 0.50 ± 0.09 a | 0.06 ± 0.04 | 0.46 ± 0.1 b | 0.09 ± 0.02 | - | - |
| 52 | 1507 | 1505 | β-Bisabolene | - | - | 0.75 ± 0.09 a | 0.13 ± 0.04 | - | - |
| 53 | 1509 | 1515 | β-Curcumene | 0.12 ± 0.02 b | 0.19 ± 0.08 a | - | - | - | - |
| 54 | 1538 | 1522 | β-Sesquiphellandrene | - | - | 2.16 ± 0.05 a | 0.04 ± 0.01 c | 0.10 ± 0.02 b | 0.08 ± 0.03 |
| 55 | 1548 | 1546 | Selina-3,7(11)-diene | 0.54 ± 0.09 a | 0.18 ± 0.07 | - | - | 0.07 ± 0.01 b | 0.09 ± 0.02 |
| 56 | 1569 | 1549 | Elemol | - | - | 0.19 ± 0.07 | 0.34 ± 0.05 c | 0.73 ± 0.1 a | 0.65 ± 0.08 b |
| 57 | 1572 | 1561 | Germacrene B | 0.26 ± 0.05 b | 0.52 ± 0.09 a | - | - | - | - |
| 58 | 1578 | 1563 | (E)-Nerolidol | - | 0.33 ± 0.07 a | 0.09 ± 0.02 | 0.12 ± 0.01 b | 0.06 ± 0.01 c | |
| 59 | 1580 | 1575 | Germacrene D-4-ol | 0.12 ± 0.04 | 0.37 ± 0.08 a | - | - | 0.27 ± 0.10 b | 0.15 ± 0.07 c |
| 60 | 1581 | 1578 | Spathulenol | - | - | 0.09 ± 0.02 | 0.07 ± 0.01 a | - | - |
| 61 | 1586 | 1583 | Caryophyllene oxide | - | 0.29 ± 0.05 a | 0.05 ± 0.01 d | 0.20 ± 0.04 b | 0.10 ± 0.02 c | |
| 62 | 1594 | 1606 | Curzerenone | 40.60 ± 2.46 c | 26.47 ± 1.88 d | 2.22 ± 0.47 f | 7.14 ± 1.88 e | 56.34 ± 3.62 b | 62.81 ± 4.12 a |
| 63 | 1605 | 1607 | 5-epi-7-epi-α-Eudesmol | 0.11 ± 0.03 | 0.08 ± 0.02 a | - | - | - | - |
| 64 | 1607 | 1608 | β-Atlantol | 0.78 ± 0.14 a | 0.18 ± 0.02 b | - | - | - | - |
| 65 | 1611 | 1608 | Humulene epoxide II | - | - | 0.76 ± 0.14 a | 0.09 ± 0.02 d | 0.49 ± 0.09 c | 0.65 ± 0.13 b |
| 66 | 1623 | 1619 | 1,10-di-epi-Cubenol | - | - | 0.22 ± 0.05 a | 0.13 ± 0.03 b | 0.13 ± 0.04 b | 0.06 ± 0.01 c |
| 67 | 1631 | 1631 | Muurola-4,10(14)-dien-1-β-ol | 0.27 ± 0.04 a | 0.09 ± 0.02 b | - | - | - | - |
| 68 | 1632 | 1632 | γ-Eudesmol | - | - | - | - | 0.31 ± 0.10 a | 0.07 ± 0.03 b |
| 69 | 1646 | 1646 | Cubenol | 0.51 ± 0.09 b | 0.17 ± 0.04 b | 1.29 ± 0.24 a | 0.25 ± 0.08 b | 1.28 ± 0.44 a | 0.63 ± 0.18 b |
| 70 | 1648 | 1650 | β-Eudesmol | - | - | 0.20 ± 0.05 | 0.16 ± 0.04 a | - | - |
| 71 | 1654 | 1653 | α-Eudesmol | 0.32 ± 0.10 a | 0.12 ± 0.02 b | - | - | 0.11 ± 007 b | 0.12 ± 0.06 |
| 72 | 1659 | 1669 | ar-Turmerone | - | - | - | - | 0.17 ± 0.08 c | 0.29 ± 0.07 a |
| 73 | 1667 | 1675 | β-Bisabolol | 0.17 ± 0.03 a | 0.12 ± 0.02 b | - | - | 0.09 ± 0.04 c | 0.07 ± 0.03 c |
| 74 | 1670 | 1677 | (Z)-Nerolidyl acetate | - | - | 0.12 ± 0.05 | 0.05 ± 0.01 a | - | - |
| 75 | 1684 | 1693 | Germacrone | 8.63 ± 1.21 c | 12.35 ± 2.04 b | 6.69 ± 1.78 d | 16.44 ± 3.03 a | 4.22 ± 0.78 f | 4.34 ± 1.0 e |
| 76 | 1703 | 1701 | Curlone (β-Turmerone) | - | - | 14.19 ± 2.26 a | 12.31 ± 1.55 b | - | - |
| 77 | 1715 | 1718 | Curcuphenol | - | - | 0.61 ± 0.08 a | 0.14 ± 0.01 b | - | - |
| 78 | 1716 | 1718 | (Z)-α-Atlantone | 0.16 ± 0.03 b | 0.45 ± 0.10 a | - | - | - | - |
| 79 | 1720 | 1731 | Chamzulene | - | - | - | - | 0.14 ± 0.04 | 0.11 ± 0.02 a |
| 80 | 1722 | 1733 | Zerumbone | - | - | - | - | 0.15 ± 0.04 | 0.19 ± 0.03 a |
| 81 | 1738 | 1734 | Curcumenol | 4.06 ± 0.45 a | 1.87 ± 0.98 b | 1.64 ± 0.55 d | 1.69 ± 0.25 c | - | - |
| 82 | 1754 | 1747 | Neocurdione | 0.51 ± 0.12 a | 0.23 ± 0.03 b | - | - | ||
| 83 | 1771 | 1753 | Xanthorrhizol | 0.30 ± 0.06 f | 1.19 ± 0.27 e | 6.76 ± 1.23 b | 13.07 ± 1.96 a | 6.23 ± 1.09 c | 2.84 ± 0.81 d |
| 84 | 1783 | 1778 | α-(E)-Atlantone | - | - | - | - | 1.02 ± 0.22 a | 0.40 ± 0.08 b |
| 85 | 1789 | 1784 | γ-Eudesmol acetate | - | - | - | - | 0.04 ± 0.01 | 0.35 ± 0.07 a |
| Monoterpene hydrocarbons | 15.64 ± 0.57 | 8.79 ± 0.23 | 11.45 ± 0.35 | 5.88 ± 0.12 | 3.01 ± 0.66 | 4.44 ± 0.11 | |||
| Oxygenated monoterpenes | 13.53 ± 0.84 | 33.02 ± 0.87 | 30.32 ± 0.85 | 31.13 ± 1.03 | 4.43 ± 0.28 | 5.62 ± 0.18 | |||
| Sesquiterpene hydrocarbons | 8.18 ± 0.44 | 9.46 ± 0.66 | 15.26 ± 1.12 | 5.72 ± 0.18 | 11.43 ± 0.64 | 9.78 ± 0.38 | |||
| Oxygenated sesquiterpenes | 55.76 ± 1.25 | 43.08 ± 1.02 | 36.09 ± 1.56 | 52.24 ± 1.24 | 71.78 ± 1.88 | 73.42 ± 0.56 | |||
| Others | 0.91 ± 0.11 | 0.49 ± 0.08 | - | - | 0.11 ± 0.01 | 0.56 ± 0.09 | |||
| Total | 94.02 ± 0.94 | 94.84 ± 1.64 | 93.12 ± 0.66 | 94.97 ± 0.76 | 90.76 ± 0.87 | 93.82 ± 0.35 | |||
2.3. Antioxidant Activity
2.4. Anti-Tyrosinase Activity
2.5. Anti-Cancer Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell lines and Culture Medium
3.3. Plant Materials
3.4. Extraction Methods
3.4.1. Hydrodistillation (HD)
3.4.2. Solvent Free Microwave Extraction (SFME)
3.5. Compound Identification Using GC-MS Analysis
3.6. Antioxidant Assay
3.6.1. DPPH Assay
3.6.2. ABTS Assay
3.7. Anti-Tyrosinase Assay
3.8. Anti-Cancer Activity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effect essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef]
- Nasim, N.; Ray, A.; Singh, S.; Jena, S.; Sahoo, A.; Kar, B.; Sandeep, I.S.; Mohanty, S.; Nayak, S. Characterization of Kewda volatile components by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. Nat. Prod. Res. 2017, 31, 853–856. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Asghari, G.; Mostajeran, A.; Shebli, M. Curcuminoid and essential oil components of turmeric at different stages of growth cultivated in Iran. Res. Pharm. Sci. 2010, 4, 55–61. [Google Scholar]
- Izgi, M.N.; Telci, I.; Elmastas, M. Variation in essential oil composition of coriander (Coriandrum sativum L.) varieties cultivated in two different ecologies. J. Essent. Oil Res. 2017, 29, 494–498. [Google Scholar] [CrossRef]
- Tavakolpour, Y.; Moosavi-Nasab, M.; Niakousari, M.; Haghighi-Manesh, S.; Hashemi, S.M.B.; Mousavi Khaneghah, A. Comparison of four extraction methods for essential oil from Thymus daenensis Subsp lancifolius and chemical analysis of extracted essential oil. J. Food Process. Preserv. 2017, 41, 13046. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, 1231–1249. [Google Scholar] [CrossRef]
- Sahoo, A.; Kar, B.; Jena, S.; Dash, B.; Ray, A.; Sahoo, S.; Nayak, S. Qualitative and quantitative evaluation of rhizome essential oil of eight different cultivars of Curcuma longa L. (Turmeric). J. Essent. Oil-Bear. Plants 2019, 22, 239–247. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Babu, K.N.; Sivaraman, K. Turmeric: The Genus Curcuma; CRC Press: London, UK; New York, NY, USA, 2007; pp. 1–479. [Google Scholar]
- Sasikumar, B. Genetic resources of Curcuma: Diversity, characterization and utilization. Plant Genet. Res. 2005, 3, 230–251. [Google Scholar] [CrossRef]
- Kumar, P.K.; Thomas, V.P.; Sabu, M.; Rajendran, A. Some important medicinal herbs in the family Zingiberaceae in India. Herb. Med. 2010, 65. [Google Scholar]
- Parida, R.; Mohanty, S.; Nayak, S. Chemical composition and anti-proliferative activity of essential oil from rhizomes of micropropagated Curcuma aromatica in eastern India. J. Biol. Act. Prod. Nat. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Rahmat, E.; Lee, J.; Kang, Y. Javanese turmeric (Curcuma xanthorrhiza Roxb.): Ethnobotany, phytochemistry, biotechnology, and pharmacological activities. Evid. Based Complement. Altern. Med. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Akter, R.; Hasan, S.R.; Siddiqua, S.A.; Majumder, M.M.; Hossain, M.M.; Alam, M.A.; Haque, S.; Ghani, A. Evaluation of analgesic and antioxidant potential of the leaves of Curcuma alismatifolia Gagnep. Stamford J. Pharm. Sci. 2008, 1, 3–9. [Google Scholar] [CrossRef]
- Hasan, S.R.; Hossain, M.M.; Akter, R.; Jamila, M.; Mazumder, M.E.H.; Rahman, S. DPPH free radical scavenging activity of some Bangladeshi medicinal plants. J. Med. Plants Res. 2009, 3, 875–879. [Google Scholar]
- Mau, J.L.; Lai, E.Y.; Wang, N.P.; Chen, C.C.; Chang, C.H.; Chyau, C.C. Composition and antioxidant activity of the essential oil from Curcuma zedoaria. Food Chem. 2003, 82, 583–591. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Jagan, L.; Rao, M.; Sakariah, K.K. Chemistry and biological activity of Curcuma longa. Trend Food Sci. Technol. 2005, 16, 533–548. [Google Scholar] [CrossRef]
- Lobo, R.; Prabhu, K.S.; Shirwaikar, A. Curcuma zedoaria Rosc (white turmeric): A review of its chemical, pharmacological and ethnomedicinal properties. J. Pharm. Pharmacol. 2009, 61, 13–21. [Google Scholar] [CrossRef]
- Fiorini, D.; Scortichini, S.; Bonacucina, G.; Greco, N.G.; Mazzara, E.; Petrelli, R.; Torresi, J.; Maggi, F.; Cespi, M. Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. Ind. Crops Prod. 2020, 154, 112688. [Google Scholar] [CrossRef]
- Araujo, A.R.; Périno, S.; Fernandez, X.; Cunha, C.; Rodrigues, M.; Ribeiro, M.P.; Jordao, L.; Silva, L.A.; Rodilla, J.; Coutinho, P.; et al. Solvent-free microwave extraction of Thymus Mastichina essential oil: Influence on their chemical composition and on the antioxidant and antimicrobial activities. Pharmaceuticals 2021, 14, 709. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of different isolation methods of essential oil from Citrus fruits: Cold pressing, hydrodistillation and microwave ‘dry’ distillation. Flavour Fragr. J. 2007, 22, 494–504. [Google Scholar] [CrossRef]
- Mohamadi, M.; Shamspur, T.; Mostafavi, A. Comparison of microwave-assisted distillation and conventional hydrodistillation in the essential oil extraction of flowers Rosa damascena Mill. J. Essent. Oil Res. 2013, 25, 55–56. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A. 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Aziz, N.A.A.; Hassan, J.; Osman, N.H.; Abbas, Z. Extraction of essential oils from Zingiberaceace family by using solvent-free microwave extraction (SFME), microwave-assisted extraction (MAE) and hydrodistillation (HD). Asian J. Appl. Sci. 2017, 5, 97–100. [Google Scholar]
- Filly, A.; Fernandez, X.; Minuti, M.; Visinoni, F.; Cravotto, G.; Chemat, F. Solvent free microwave extraction of essential oil from aromatic herbs: From laboratory to pilot and industrial scale. Food Chem. 2014, 150, 193–198. [Google Scholar] [CrossRef]
- Farhat, A.; Ginies, C.; Romdhane, M.; Chemat, F. Eco-friendly and cleaner process for isolation of essential oil using microwave energy: Experimental and theoretical study. J. Chromatogr. A. 2009, 1216, 5077–5085. [Google Scholar] [CrossRef]
- Qi, X.L.; Li, T.T.; Wei, Z.F.; Guo, N.; Luo, M.; Wang, W.; Zu, Y.G.; Fu, Y.J.; Peng, X. Solvent-free microwave extraction of essential oil from pigeon pea leaves [Cajanus cajan (L.) Millsp.] and evaluation of its antimicrobial activity. Ind. Crops Prod. 2014, 58, 322–328. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.; Andrés, M.A.; Labidi, J. Microwave-assisted extraction of Curcuma longa l. Oil: Optimization, chemical structure and composition, antioxidant activity and comparison with conventional soxhlet extraction. Molecules 2021, 26, 1516. [Google Scholar] [CrossRef]
- Bener, M.; Özyürek, M.; Güçlü, K.; Apak, R. Optimization of microwave-assisted extraction of curcumin from Curcuma longa L. (Turmeric) and evaluation of antioxidant activity in multi-test systems. Rec. Nat. Prod. 2016, 10, 542. [Google Scholar]
- Padmapriya, K.; Dutta, A.; Chaudhuri, S.; Dutta, D. Microwave assisted extraction of mangiferin from Curcuma amada. 3 Biotech 2012, 2, 27–30. [Google Scholar] [CrossRef]
- Theanphong, O.; Jenjittikul, T.; Mingvanish, W. Essential oils composition of nine Curcuma species from Thailand: A chemotaxonomic approach. Gard. Bull. Singapore 2019, 71, 499–518. [Google Scholar] [CrossRef]
- Theanphong, O.; Mingvanish, W. Chemical constituents and antioxidant activities of essential oils from roots and rhizomes of Curcuma alismatifolia Gagnap. from Thailand. J. Appl. Sci. 2017, 16, 105–111. [Google Scholar] [CrossRef]
- Bordoloi, A.K.; Sperkova, J.; Leclercq, P.A. Essential oils of Curcuma aromatica Salisb. from northeast India. J. Essent. Oil Res. 1999, 11, 537–540. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, L.; Yang, Z.; Chen, F.; Zheng, X.; Liu, X. Chemical compositions, antioxidative, antimicrobial, anti-inflammatory and antitumor activities of Curcuma aromatica Salisb. essential oils. Ind. Crops Prod. 2017, 108, 6–16. [Google Scholar] [CrossRef]
- Salea, R.; Widjojokusumo, E.; Veriansyah, B.; Tjandrawinata, R.R. Optimizing oil and xanthorrhizol extraction from Curcuma xanthorrhiza Roxb. rhizome by supercritical carbon dioxide. J. Food Sci. Technol. 2014, 51, 2197–2203. [Google Scholar] [CrossRef]
- Djouahri, A.; Boudarene, L.; Meklati, B.Y. Effect of extraction method on chemical composition, antioxidant and anti-inflammatory activities of essential oil from the leaves of Algerian Tetraclinis articulata (Vahl) Masters. Ind. Crops Prod. 2013, 44, 32–36. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Mahfud, M. Microwave-assisted hydrodistillation for extraction of essential oil from patchouli (Pogostemon cablin) leaves. Period. Polytech. Chem. Eng. 2017, 61, 82–92. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Okoh, O.O.; Sadimenko, A.P.; Afolayan, A.J. Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chem. 2010, 120, 308–312. [Google Scholar] [CrossRef]
- Akloul, R.; Benkaci-Ali, F.; Eppe, G. Kinetic study of volatile oil of Curcuma longa L. rhizome and Carum carvi L. fruits extracted by microwave-assisted techniques using the cryogrinding. J. Essent. Oil Res. 2014, 26, 473–485. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Setzer, W.N. Variations in the volatile compositions of Curcuma species. Foods 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Albaqami, J.J.; Hamdi, H.; Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Kuttithodi, A.M.; Famurewa, A.C.; Pathrose, B. Chemical Composition and Biological Activities of the Leaf Essential Oils of Curcuma longa, Curcuma aromatica and Curcuma angustifolia. Antibiotics 2022, 11, 1547. [Google Scholar] [CrossRef] [PubMed]
- Jarikasem, S.; Thubthimthed, S.; Chawananoraseth, K.; Suntorntanasat, T. Essential oils from three Curcuma species collected in Thailand. In III WOCMAP Congress on Medicinal and Aromatic Plants; Bernath, J., Nemeth, E., Craker, L.E., Gardner, Z.E., Eds.; Acta Horticulturae: Chiang Mai, Thailand, 2003; Volume 3, pp. 37–41. [Google Scholar] [CrossRef]
- Jantan, I.; Saputri, F.C.; Qaisar, M.N.; Buang, F. Correlation between chemical composition of Curcuma domestica and Curcuma xanthorrhiza and their antioxidant effect on human low-density lipoprotein oxidation. Evid. Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Kojima, H.; Yanai, T.; Toyota, A. Essential oil constituents from Japanese and Indian Curcuma aromatica rhizomes. Planta med. 1998, 64, 380–381. [Google Scholar] [CrossRef]
- Ab Halim, M.R.; Tan, M.S.M.Z.; Ismail, S.; Mahmud, R. Standardization and phytochemical studies of Curcuma xanthorrhiza Roxb. Int. J. Pharm. Pharm. Sci. 2012, 4, 606–610. [Google Scholar]
- Akter, R.; Hasan, S.R.; Hossain, M.M.; Jamila, M.; Chowdhury, S.S.; Mazumder, M.E.H.; Rahman, S. Antidiarrhoeal and antioxidant properties of Curcuma alismatifolia leaves. Aust. J. Basic Appl. Sci. 2010, 4, 450–456. [Google Scholar]
- Tsai, S.Y.; Huang, S.J.; Chyau, C.C.; Tsai, C.H.; Weng, C.C.; Mau, J.L. Composition and antioxidant properties of essential oils from Curcuma rhizome. Asian J. Arts Sci. 2011, 2, 57–66. [Google Scholar] [CrossRef]
- Al-Reza, S.M.; Rahman, A.; Sattar, M.A.; Rahman, M.O.; Fida, H.M. Essential oil composition and antioxidant activities of Curcuma aromatica Salisb. Food Chem. Toxicol. 2010, 48, 1757–1760. [Google Scholar] [CrossRef]
- Nurrulhidayah, A.F.; Rafi, M.; Lukitaningsih, E.; Widodo, H.; Rohman, A.; Windarsih, A. Review on in vitro antioxidant activities of Curcuma species commonly used as herbal components in Indonesia. Food Res. 2020, 4, 286–293. [Google Scholar]
- Kusumadewi, A.P.; Martien, R.; Pramono, S.; Setyawan, A.A.; Windarsih, A.; Rohman, A. Application of FTIR spectroscopy and chemometrics for correlation of antioxidant activities, phenolics and flavonoid contents of Indonesian Curcuma xanthorrhiza. Int. J. Food Prop. 2022, 25, 2364–2372. [Google Scholar] [CrossRef]
- Joshi, S.C.; Mathela, C.S. Antioxidant and antibacterial activities of the leaf essential oil and its constituents furanodienone and curzerenone from Lindera pulcherrima (Nees.) Benth. ex Hook. f. Pharmacogn Res. 2012, 4, 80. [Google Scholar]
- Li, Y.; Li, S.; Lin, S.J.; Zhang, J.J.; Zhao, C.N.; Li, H.B. Microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris Fruit: Optimization and identification of phenolic compounds. Molecules 2017, 22, 1481. [Google Scholar] [CrossRef] [PubMed]
- Berka-Zougali, B.; Ferhat, M.A.; Hassani, A.; Chemat, F.; Allaf, K.S. Comparative study of essential oils extracted from Algerian Myrtus communis L. leaves using microwaves and hydrodistillation. Int. J. Mol. Sci. 2012, 13, 4673–4695. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Carrera, M. New and experimental treatments of cloasma and other hypermelanoses. Dermatol. Clin. 2007, 25, 353–362. [Google Scholar] [CrossRef]
- Chao, W.W.; Su, C.C.; Peng, H.Y.; Chou, S.T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef]
- Mustarichie, R.; Levita, J.; Febriani, D. In-silico study of curcumin, demethoxycurcumin, and xanthorrizol as skin whitening agents. World J. Pharm. Sci. 2013, 1, 72–80. [Google Scholar]
- Misra, B.B.; Dey, S. TLC-bioautographic evaluation of in vitro anti-tyrosinase and anti-cholinesterase potentials of sandalwood oil. Nat. Prod. Commun. 2013, 8, 253–256. [Google Scholar] [CrossRef]
- Rachkeeree, A.; Kantadoung, K.; Puangpradub, R.; Suksathan, R. Phytochemicals, antioxidants and anti-tyrosinase analyses of selected ginger plants. Pharmacogn. J. 2020, 12, 872–883. [Google Scholar] [CrossRef]
- Firmansyah, D.; Sumiwi, S.A.; Saptarini, N.M.; Levita, J. Molecular interaction of curcumin, demethoxycurcumin, bisdemethoxycurcumin, and turmerone of Curcuma longa with tyrosinase and tyrosinase-related protein-1. Rasayan J. Chem. 2021, 14, 2298–2303. [Google Scholar] [CrossRef]
- Kim, J.A.; Son, J.K.; Chang, H.W.; Jahng, Y.; Kim, Y.; Na, M.; Lee, S.H. Inhibition of mushroom tyrosinase and melanogenesis B16 mouse melanoma cells by components isolated from Curcuma longa. Nat. Prod. Commun. 2008, 3, 1655–1658. [Google Scholar] [CrossRef]
- Lee, Y.K.; Song, Y.S.; Lee, K.K. Evaluation of antioxidant, tyrosinase inhibitory and anti-inflammatory activities of turmeric (Curcuma longa) extracts. Int. J. Beauty Cult. Arts 2012, 1, 2–8. [Google Scholar]
- An, B.J.; Lee, J.Y.; Park, T.S.; Pyeon, J.R.; Bae, H.J.; Song, M.I.; Baek, E.J.; Park, J.M.; Son, J.H.; Lee, C.E.; et al. Antioxidant Activity and Whitening Effect of Extraction Conditions in Curcuma longa L. Korean J. Med. Crop Sci. 2006, 14, 168–172. [Google Scholar]
- Rijo, P.; Falé, P.L.; Serralheiro, M.L.; Simões, M.F.; Gomes, A.; Reis, C. Optimization of medicinal plant extraction methods and their encapsulation through extrusion technology. Measurement 2014, 58, 249–255. [Google Scholar] [CrossRef]
- El Maaiden, E.; Bouzroud, S.; Nasser, B.; Moustaid, K.; El Mouttaqi, A.; Ibourki, M.; Boukcim, H.; Hirich, A.; Kouisni, L.; El Kharrassi, Y. A comparative study between conventional and advanced extraction techniques: Pharmaceutical and cosmetic properties of plant extracts. Molecules 2022, 27, 2074. [Google Scholar] [CrossRef]
- Elhawary, S.S.; El Tantawy, M.E.; Rabeh, M.A.; Fawaz, N.E. DNA fingerprinting, chemical composition, antitumor and antimicrobial activities of the essential oils and extractives of four Annona species from Egypt. DNA 2013, 3, 59–68. [Google Scholar]
- Zare, E.; Jamali, T.; Ardestani, S.K.; Kavoosi, G. Synergistic effect of Zataria Multiflora essential oil on doxorubicin-induced growth inhibition of PC3 cancer cells and apoptosis. Complement. Ther. Clin. Pract. 2021, 42, 101286. [Google Scholar] [CrossRef] [PubMed]
- Khruengsai, S.; Sripahco, T.; Rujanapun, N.; Charoensup, R.; Pripdeevech, P. Chemical composition and biological activity of Peucedanum dhana A. Ham essential oil. Sci. Rep. 2021, 11, 19079. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, Y.; Hsi, Y.-T.; Chang, C.-S.; Huang, L.-F.; Ho, C.-T.; Way, T.-D.; Kao, J.-Y. Chemical constituents and anticancer activity of Curcuma zedoaria Roscoe essential oil against non-small cell lung carcinoma cells in vitro and in vivo. J. Agric. Food Chem. 2013, 61, 11418–11427. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Zhang, J.; Zhang, X.; Martin, R.C. Hepatic protection and anticancer activity of curcuma: A potential chemopreventive strategy against hepatocellular carcinoma. Int. J. Oncol. 2014, 44, 505–513. [Google Scholar] [CrossRef]
- Ma, J.-W.; Tsao, T.C.-Y.; Hsi, Y.-T.; Lin, Y.-C.; Chen, Y.; Chen, Y.; Ho, C.-T.; Kao, J.-Y.; Way, T.-D. Essential oil of Curcuma aromatica induces apoptosis in human non-small-cell lung carcinoma cells. J. Funct. Foods. 2016, 22, 101–112. [Google Scholar] [CrossRef]
- Simamora, A.; Timotius, K.H.; Yerer, M.B.; Setiawan, H.; Mun'im, A. Xanthorrhizol, a potential anticancer agent, from Curcuma xanthorrhiza Roxb. Phytomedicine 2022, 105, 154359. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Fang, B.; Ma, F.; Zheng, Q.; Deng, P.; He, G. Anti-tumor effect of germacrone on human hepatoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Eur. J. Pharmacol. 2013, 698, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.; Eltayeb, N.M.; Khairuddean, M.; Salhimi, S.M. Bioactive chemical constituents from Curcuma caesia Roxb. rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231. Nat. Prod. Res. 2021, 35, 3166–3170. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, Y.; Greenberg, S.A.; Jenkins, B.A.; Marshall, K.L.; Dimitrov, L.V.; Nelson, A.M.; Owens, D.M.; Lumpkin, E.A. Camphor white oil induces tumor regression through cytotoxic T cell-dependent mechanisms. Mol. Carcinog. 2019, 58, 722–734. [Google Scholar] [CrossRef]
- Rahaman, A.; Chaudhuri, A.; Sarkar, A.; Chakraborty, S.; Bhattacharjee, S.; Mandal, D.P. Eucalyptol targets PI3K/Akt/mTOR pathway to inhibit skin cancer metastasis. Carcinogenesis 2022, 43, 571–583. [Google Scholar] [CrossRef]
- Sampath, S.; Veeramani, V.; Krishnakumar, G.S.; Sivalingam, U.; Madurai, S.L.; Chellan, R. Evaluation of in vitro anticancer activity of 1,8-Cineole-containing n-hexane extract of Callistemon citrinus (Curtis) Skeels plant and its apoptotic potential. Biomed. Pharmacother. 2017, 93, 296–307. [Google Scholar] [CrossRef]
- Aydin, E.; Türkez, H.; Ta¸sdemir, S. Anticancer and antioxidant properties of terpinolene in rat brain cells. Arch. Ind. Hyg. Toxicol. 2013, 64, 415–424. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.S.; Salman, M.S.; Fadl, M.A.; Elkhateeb, A.; El-Awady, M.A. Chemical composition of hydrodistillation and solvent free microwave extraction of essential oils from Mentha piperita L. growing in Taif, Kingdom of Saudi Arabia, and their anticancer and antimicrobial activity. Orient. J. Chem. 2018, 34, 222. [Google Scholar] [CrossRef]
- Zhu, J.J.; Yang, J.J.; Wu, G.J.; Jiang, J.G. Comparative antioxidant, anticancer and antimicrobial activities of essential oils from Semen Platycladi by different extraction methods. Ind Crops Prod. 2020, 146, 112206. [Google Scholar] [CrossRef]
- Chang, L.W.; Juang, L.J.; Wang, B.S.; Wang, M.Y.; Tai, H.M.; Hung, W.J.; Chen, Y.J.; Huang, M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2011, 49, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Kamila, P.K.; Ray, A.; Jena, S.; Mohapatra, P.K.; Panda, P.C. Chemical composition and antioxidant activities of the essential oil of Hypericum gaitii Haines–an endemic species of Eastern India. Nat. Prod. Res. 2018, 32, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.M.; Rahmani, M.; Ee, G.C.L.; Sukari, M.A.; Yahayu, M.; Amin, M.A.M.; Ali, A.M.; Go, R. Antioxidant, antimicrobial and tyrosinase inhibitory activities of xanthones isolated from Artocarpus obtusus FM Jarrett. Molecules 2012, 17, 6071–6082. [Google Scholar] [CrossRef] [PubMed]
- Basappa, G.; Kumar, V.; Sarojini, B.K.; Poornima, D.V.; Gajula, H.; Sannabommaji, T.K.; Rajashekar, J. Chemical composition, biological properties of Anisomeles indica Kuntze essential oil. Ind. Crops Prod. 2015, 77, 89–96. [Google Scholar] [CrossRef]
- Manosroi, J.; Pongsathorn, D.; Aranya, M. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef]









| Samples | Extraction Time (h) | Energy Consumption (kWh) | Colour of Essential Oil | Water (mL/100 g) | ||||
|---|---|---|---|---|---|---|---|---|
| HD | SFME | HD | SFME | HD | SFME | HD | SFME | |
| C. alismatifolia | 7 | 4 | 9.43 | 2.13 | Pale Yellow | Deep Yellow | 1000 | 100 |
| C. aromatica | 7 | 4 | 9.43 | 2.13 | Deep brown | Light brown | 1000 | 100 |
| C. xanthorrhiza | 7 | 4 | 9.43 | 2.13 | Pale yellow | Pale yellow | 1000 | 100 |
| Samples | IC50 Value (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| MCF7 | HepG2 | PC3 | 3T3-L1 | |||||
| HD | SFME | HD | SFME | HD | SFME | HD | SFME | |
| C. alismatifolia | 55.62 ± 0.89 a | 52.86 ± 1.23 b | 111.89 ± 2.45 a | 108.97 ± 2.10 b | 102.24 ± 2.56 a | 98.61 ± 1.87 b | 540.62 ± 4.32 b | 551.23 ± 5.12 a |
| C. aromatica | 115.89 ± 1.83 a | 108.87 ± 1.07 b | 140.58 ± 1.04 a | 139.41 ± 1.61 a | 71.67 ± 1.89 a | 69.92 ± 1.44 a | 745.89 ± 9.10 b | 761.04 ± 8.32 a |
| C. xanthorrhiza | 129.78 ± 1.92 a | 115.57 ± 1.58 b | 191.49 ± 1.48 a | 187.94 ± 1.78 b | 97.28 ± 2.81 a | 94.22 ± 1.98 b | 626.54 ± 8.90 b | 639.48 ± 5.88 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, S.; Ray, A.; Naik, P.K.; Sahoo, A.; Jena, S.; Das, P.K.; Patnaik, J.; Panda, P.C.; Nayak, S. Variation in Yield, Chemical Composition and Biological Activities of Essential Oil of Three Curcuma Species: A Comparative Evaluation of Hydrodistillation and Solvent-Free Microwave Extraction Methods. Molecules 2023, 28, 4434. https://doi.org/10.3390/molecules28114434
Mohanty S, Ray A, Naik PK, Sahoo A, Jena S, Das PK, Patnaik J, Panda PC, Nayak S. Variation in Yield, Chemical Composition and Biological Activities of Essential Oil of Three Curcuma Species: A Comparative Evaluation of Hydrodistillation and Solvent-Free Microwave Extraction Methods. Molecules. 2023; 28(11):4434. https://doi.org/10.3390/molecules28114434
Chicago/Turabian StyleMohanty, Swagat, Asit Ray, Pradeep Kumar Naik, Ambika Sahoo, Sudipta Jena, Prabhat Kumar Das, Jeetendranath Patnaik, Pratap Chandra Panda, and Sanghamitra Nayak. 2023. "Variation in Yield, Chemical Composition and Biological Activities of Essential Oil of Three Curcuma Species: A Comparative Evaluation of Hydrodistillation and Solvent-Free Microwave Extraction Methods" Molecules 28, no. 11: 4434. https://doi.org/10.3390/molecules28114434
APA StyleMohanty, S., Ray, A., Naik, P. K., Sahoo, A., Jena, S., Das, P. K., Patnaik, J., Panda, P. C., & Nayak, S. (2023). Variation in Yield, Chemical Composition and Biological Activities of Essential Oil of Three Curcuma Species: A Comparative Evaluation of Hydrodistillation and Solvent-Free Microwave Extraction Methods. Molecules, 28(11), 4434. https://doi.org/10.3390/molecules28114434