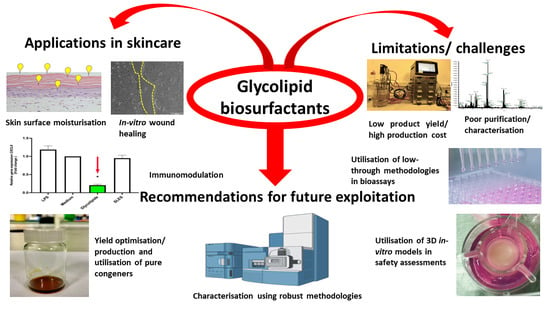
Glycolipid Biosurfactants in Skincare Applications: Challenges and Recommendations for Future Exploitation
Abstract
:1. Introduction
2. Glycolipid Biosurfactants as Promising Alternatives to Synthetic Surfactants
2.1. Adverse Effects of Synthetic Surfactants on the Environment and Consumer Skin Health
2.2. Effects of Glycolipid Biosurfactants on Various Human Skin Cell Types
2.3. Effects of Glycolipid Biosurfactants on the Human Skin Microbiome
3. Challenges and Recommendations for Assessing the Potential Use of Glycolipid Biosurfactants in Skincare Applications
3.1. Pathogenicity of Glycolipid-Producing Strains, Low Product Yield, Cost of Large-Scale Production, and Limited Structural Variability
3.2. Utilisation of Impure/Poorly Characterised Glycolipid Biosurfactant Congeners in Bioassays
3.3. Limited In Vitro Studies on Potential Benefits of Glycolipids to the Human Skin and the Skin Microbiome and Treatment Conditions
3.4. Utilisation of 2D In Vitro Cell Cultures in Bioassays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, L.A.; Da, M.; Silva, G.C.; José, I.; Durval, B.; Gercyane, K.; Bezerra, O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; et al. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Roy, A.A. Review on the Biosurfactants: Properties, Types and Its Applications. J. Fundam. Renew. Energy Appl. 2017, 8, 1000248. [Google Scholar] [CrossRef]
- Winterburn, J.B.; Martin, P.J. Foam mitigation and exploitation in biosurfactant production. Biotechnol. Lett. 2012, 34, 187–195. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—A new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Sharma, D.; Singh Saharan, B. Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnol. Rep. 2016, 11, 27–35. [Google Scholar] [CrossRef]
- Wongsirichot, P.; Ingham, B.; Winterburn, J. A review of sophorolipid production from alternative feedstocks for the development of a localized selection strategy. J. Clean. Prod. 2021, 319, 128727. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Natural surfactants used in cosmetics: Glycolipids. Int. J. Cosmet. Sci. 2009, 31, 255–261. [Google Scholar] [CrossRef]
- Dardouri, M.; Mendes, R.M.; Frenzel, J.; Costa, J.; Ribeiro, I.A.C. Seeking faster, alternative methods for glycolipid biosurfactant characterization and purification. Anal. Bioanal. Chem. 2021, 413, 4311–4320. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Bogaert, I.N.A. Van Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Ghosh, T.K.; Das, N. Application of Bio-Surfactants in Cosmetics and Pharmaceutical Industry. Sch. Acad. J. Pharm. 2017, 6, 320–329. [Google Scholar] [CrossRef]
- Nian Tan, Y.; Li, Q. Microbial production of rhamnolipids using sugars as carbon sources. Microb. Cell Fact. 2018, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, L.; Rajasree, S. Biosurfactant: Pharmaceutical Perspective. J. Anal. Pharm. Res. 2017, 4, 11–12. [Google Scholar] [CrossRef]
- Shu, Q.; Lou, H.; Wei, T.; Liu, X.; Chen, Q. Contributions of Glycolipid Biosurfactants and Glycolipid-Modified Materials to Antimicrobial Strategy: A Review. Pharmaceutics 2021, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Perfumo, A.; Rancich, I.; Banat, I.M. Possibilities and challenges for biosurfactants use in petroleum industry. Adv. Exp. Med. Biol. 2010, 672, 135–145. [Google Scholar] [CrossRef]
- Banat, I.M.; Desai, J.D. 97/02677 Microbial production of surfactants and their commercial potential. Fuel Energy Abstr. 1997, 38, 221. [Google Scholar] [CrossRef]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Characterisation of cytotoxicity and immunomodulatory effects of glycolipid biosurfactants on human keratinocytes. Appl. Microbiol. Biotechnol. 2023, 107, 137–152. [Google Scholar] [CrossRef]
- Inès, M.; Dhouha, G. Glycolipid biosurfactants: Main properties and potential applications in agriculture and food industry. J. Sci. Food Agric. 2016, 96, 4310–4320. [Google Scholar] [CrossRef]
- Jezierska, S.; Claus, S.; Van Bogaert, I. Yeast glycolipid biosurfactants. FEBS Lett. 2018, 592, 1312–1329. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Kovacic, F.; Müller, M.M.; Gerlitzki, M.; Santiago-Schübel, B.; Hofmann, D.; Tiso, T.; Blank, L.M.; Henkel, M.; Hausmann, R.; et al. Novel insights into biosynthesis and uptake of rhamnolipids and their precursors. Appl. Microbiol. Biotechnol. 2017, 101, 2865. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Rosenau, F. Heterologous Rhamnolipid Biosynthesis: Advantages, Challenges, and the Opportunity to Produce Tailor-Made Rhamnolipids. Front. Bioeng. Biotechnol. 2020, 8, 1263. [Google Scholar] [CrossRef]
- Dolman, B.M.; Kaisermann, C.; Martin, P.J.; Winterburn, J.B. Integrated sophorolipid production and gravity separation. Process Biochem. 2017, 54, 162–171. [Google Scholar] [CrossRef]
- Cho, W.Y.; Ng, J.F.; Yap, W.H.; Goh, B.H. Sophorolipids—Bio-Based Antimicrobial Formulating Agents for Applications in Food and Health. Molecules 2022, 27, 5556. [Google Scholar] [CrossRef]
- Thakur, P.; Saini, N.K.; Kumar Thakur, V.; Kumar Gupta, V.; Saini, R.V.; Saini, A.K. Rhamnolipid the Glycolipid Biosurfactant: Emerging trends and promising strategies in the field of biotechnology and biomedicine. Microb. Cell Fact. 2021, 20, 1. [Google Scholar] [CrossRef]
- Maeng, Y.; Kim, K.T.; Zhou, X.; Jin, L.; Kim, K.S.; Kim, Y.H.; Lee, S.; Park, J.H.; Chen, X.; Kong, M.; et al. A novel microbial technique for producing high-quality sophorolipids from horse oil suitable for cosmetic applications. Microb. Biotechnol. 2018, 11, 917–929. [Google Scholar] [CrossRef]
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef]
- Li, Z.; Dai, L.; Wang, D.; Mao, L.; Gao, Y. Stabilization and Rheology of Concentrated Emulsions Using the Natural Emulsifiers Quillaja Saponins and Rhamnolipids. J. Agric. Food Chem. 2018, 66, 3922–3929. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef]
- Voulgaridou, G.P.; Mantso, T.; Anestopoulos, I.; Klavaris, A.; Katzastra, C.; Kiousi, D.E.; Mantela, M.; Galanis, A.; Gardikis, K.; Banat, I.M.; et al. Toxicity profiling of biosurfactants produced by novel marine bacterial strains. Int. J. Mol. Sci. 2021, 22, 2383. [Google Scholar] [CrossRef]
- Urzedo, C.A.; Freitas, Q.; Akemi, V.; Silveira, I.; Pedrine, M.A.; Celligoi, C. Antimicrobial Applications of Sophorolipid from Candida bombicola: A Promising Alternative to Conventional Drugs. Adv. Biotechnol. Microbiol. 2018, 9, 555753. [Google Scholar]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure. Pharmaceutics 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.; Magri, A.; Baldo, C.; Camilios-Neto, D.; Minucelli, T.; Pedrine, M.A.; Celligoi, C. Review: Sophorolipids A Promising Biosurfactant and it’s Applications. Int. J. Adv. Biotechnol. Res. 2015, 6, 161–174. [Google Scholar]
- Rudden, M.; Tsauosi, K.; Marchant, R.; Banat, I.M.; Smyth, T.J. Development and validation of an ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for the quantitative determination of rhamnolipid congeners. Appl. Microbiol. Biotechnol. 2015, 99, 9177–9187. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.; Twigg, M.S.; Baccile, N.; Van Bogaert, I.N.A.; Marchant, R.; Mitchell, C.A.; Banat, I.M. Microbial sophorolipids inhibit colorectal tumour cell growth in vitro and restore haematocrit in Apcmin+/− mice. Appl. Microbiol. Biotechnol. 2022, 106, 6003–6016. [Google Scholar] [CrossRef]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef]
- Hirata, Y.; Igarashi, K.; Ueda, A.; Quan, G.L. Enhanced sophorolipid production and effective conversion of waste frying oil using dual lipophilic substrates. Biosci. Biotechnol. Biochem. 2021, 85, 1763–1771. [Google Scholar] [CrossRef]
- Dolman, B.M.; Wang, F.; Winterburn, J.B. Integrated production and separation of biosurfactants. Process Biochem. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Eslami, P.; Hajfarajollah, H.; Bazsefidpar, S. Recent advancements in the production of rhamnolipid biosurfactants by Pseudomonas aeruginosa. RSC Adv. 2020, 10, 34014–34032. [Google Scholar] [CrossRef] [PubMed]
- Manikkasundaram, V.; Baskaran, A.; Kaari, M.; Vignesh, I.; Gopikrishnan Venugopal, A.I.; Manikkam, R. Production and characterization of glycolipid biosurfactant from Streptomyces enissocaesilis HRB1 and its evaluation for biomedical and bioremediation applications. J. Surfactants Deterg. 2022. [Google Scholar] [CrossRef]
- Ahmadi-Ashtiani, H.R.; Baldisserotto, A.; Cesa, E.; Manfredini, S.; Zadeh, H.S.; Gorab, M.G.; Khanahmadi, M.; Zakizadeh, S.; Buso, P.; Vertuani, S. Microbial biosurfactants as key multifunctional ingredients for sustainable cosmetics. Cosmetics 2020, 7, 46. [Google Scholar] [CrossRef]
- Mijaljica, D.; Spada, F.; Harrison, I.P. Skin Cleansing without or with Compromise: Soaps and Syndets. Molecules 2022, 27, 2010. [Google Scholar] [CrossRef] [PubMed]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef] [PubMed]
- Bahia, F.M.; De Almeida, G.C.; De Andrade, L.P.; Campos, C.G.; Queiroz, L.R.; Da Silva, R.L.V.; Abdelnur, P.V.; Corrêa, J.R.; Bettiga, M.; Parachin, N.S. Rhamnolipids production from sucrose by engineered Saccharomyces cerevisiae. Sci. Rep. 2018, 8, 2905. [Google Scholar] [CrossRef] [PubMed]
- Durval, I.J.B.; da Silva, I.A.; Sarubbo, L.A. Application of Microbial Biosurfactants in the Food Industry. In Microbial Biosurfactants. Environmental and Microbial Biotechnology; Inamuddin, Ahamed, M.I., Prasad, R., Eds.; Springer: Singapore, 2021; Volume 112, pp. 1–10. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between surfactants and the skin—Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Corazza, M.; Lauriola, M.; Zappaterra, M.; Bianchi, A.; Virgili, A. Surfactants, skin cleansing protagonists. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1–6. [Google Scholar] [CrossRef]
- Holland, K.T.; Bojar, R.A. Cosmetics What is Their Influence on the Skin Microflora? J. Clin. Dermatol. 2002, 3, 445–449. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Chatterjee, A.; Chatterjee, S.; Saha, N.C. Acute toxicity and sublethal effects of sodium laureth sulfate on oxidative stress enzymes in benthic oligochaete worm, Tubifex tubifex. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 243, 108998. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, A.E.; Joshi, S.J.; Al-Wahaibi, Y.M.; Al-Bemani, A.S.; Al-Bahry, S.N.; Al-Maqbali, D.; Banat, I.M. Sophorolipids production by Candida bombicola ATCC 22214 and its potential application in microbial enhanced oil recovery. Front. Microbiol. 2015, 6, 1324. [Google Scholar] [CrossRef]
- Bautista-Toledo, M.I.; Rivera-Utrilla, J.; Méndez-Díaz, J.D.; Sánchez-Polo, M.; Carrasco-Marín, F. Removal of the surfactant sodium dodecylbenzenesulfonate from water by processes based on adsorption/bioadsorption and biodegradation. J. Colloid Interface Sci. 2014, 418, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mei-Hsia Chan, M.; Tan, L.S.; Leow, Y.H.; Teik-Jin Goon, A.; Goh, C.L. Comparison of Irritancy Potential of Sodium Lauryl Sulfate-free Aqueous Cream to Other Moisturizers: An Intraindividual Skin Occlusive Study. J. Clin. Aesthet. Dermatol. 2019, 12, 52. [Google Scholar]
- Leoty-Okombi, S.; Gillaizeau, F.; Leuillet, S.; Douillard, B.; Le Fresne-Languille, S.; Carton, T.; De Martino, A.; Moussou, P.; Bonnaud-Rosaye, C.; André, V. Effect of Sodium Lauryl Sulfate (SLS) Applied as a Patch on Human Skin Physiology and Its Microbiota. Cosmetics 2021, 8, 6. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17, 16–25. [Google Scholar] [CrossRef]
- Orton, D.I.; Wilkinson, J.D. Cosmetic allergy: Incidence, diagnosis, and management. Am. J. Clin. Dermatol. 2004, 5, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lydon, H.L.; Baccile, N.; Callaghan, B.; Marchant, R.; Mitchell, C.A.; Banat, I.M. Adjuvant antibiotic activity of acidic sophorolipids with potential for facilitating wound healing. Antimicrob. Agents Chemother. 2017, 61, e02547-16. [Google Scholar] [CrossRef]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A brief overview. Clin. Med. 2021, 21, 170. [Google Scholar] [CrossRef]
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 342. [Google Scholar] [CrossRef]
- Harless, W.W. Revisiting perioperative chemotherapy: The critical importance of targeting residual cancer prior to wound healing. BMC Cancer 2009, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Hipler, U.C.; Elsner, P.; Tittelbach, J. Keratinocyte and Fibroblast Wound Healing In Vitro Is Repressed by Non-Optimal Conditions but the Reparative Potential Can Be Improved by Water-Filtered Infrared A. Biomedicines 2021, 9, 1802. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Rizzuto, A.; Rossi, A.; Perri, P.; Barbetta, A.; Abdalla, K.; Caroleo, S.; Longo, C.; Amantea, B.; Sammarco, G.; et al. Skin grafting for the treatment of chronic leg ulcers—A systematic review in evidence-based medicine. Int. Wound J. 2017, 14, 149–157. [Google Scholar] [CrossRef]
- Glady, A.; Vandebroek, A.; Yasui, M. Human keratinocyte-derived extracellular vesicles activate the MAPKinase pathway and promote cell migration and proliferation in vitro. Inflamm. Regen. 2021, 41, 4. [Google Scholar] [CrossRef] [PubMed]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Gupta, P.L.; Rajput, M.; Oza, T.; Trivedi, U.; Sanghvi, G. Eminence of microbial products in cosmetic industry. Nat. Products Bioprospect. 2019, 9, 267–278. [Google Scholar] [CrossRef]
- Twigg, M.S.; Tripathi, L.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Identification and characterisation of short chain rhamnolipid production in a previously uninvestigated, non-pathogenic marine pseudomonad. Appl. Microbiol. Biotechnol. 2018, 102, 8537–8549. [Google Scholar] [CrossRef]
- Tripathi, L.; Twigg, M.S.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Biosynthesis of rhamnolipid by a Marinobacter species expands the paradigm of biosurfactant synthesis to a new genus of the marine microflora. Microb. Cell Fact. 2019, 18, 164. [Google Scholar] [CrossRef]
- Haque, E.; K Kayalvizhi, K.; Hassan, S. Biocompatibility, Antioxidant and Anti-Infective Effect of Biosurfactant Produced by Marinobacter litoralis MB15. Int. J. Pharm. Investig. 2020, 10, 173–178. [Google Scholar] [CrossRef]
- Drakontis, C.E.; Amin, S. Design of sustainable lip gloss formulation with biosurfactants and silica particles. Int. J. Cosmet. Sci. 2020, 42, 573–580. [Google Scholar] [CrossRef]
- Findley, K.; Grice, E.A. The Skin Microbiome: A Focus on Pathogens and Their Association with Skin Disease. PLoS Pathog. 2014, 10, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Pistone, D.; Meroni, G.; Panelli, S.; D’auria, E.; Acunzo, M.; Pasala, A.R.; Zuccotti, G.V.; Bandi, C.; Drago, L. A Journey on the Skin Microbiome: Pitfalls and Opportunities. Int. J. Mol. Sci. 2021, 22, 9846. [Google Scholar] [CrossRef]
- Ederveen, T.H.A.; Smits, J.P.H.; Boekhorst, J.; Schalkwijk, J.; van den Bogaard, E.H.; Zeeuwen, P.L.J.M. Skin microbiota in health and disease: From sequencing to biology. J. Dermatol. 2020, 47, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Araviiskaia, E.; Berardesca, E.; Gontijo, G.; Sanchez Viera, M.; Xiang, L.F.; Martin, R.; Bieber, T. Microbiome in healthy skin, update for dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047. [Google Scholar] [CrossRef]
- Luna, P.C. Skin Microbiome as Years Go By. Am. J. Clin. Dermatol. 2020, 21, 12. [Google Scholar] [CrossRef]
- Bouslimani, A.; da Silva, R.; Kosciolek, T.; Janssen, S.; Callewaert, C.; Amir, A.; Dorrestein, K.; Melnik, A.V.; Zaramela, L.S.; Kim, J.-N.; et al. The impact of skin care products on skin chemistry and microbiome dynamics. BMC Biol. 2019, 17, 47. [Google Scholar] [CrossRef]
- Prescott, S.; Reiche, L. Skin microbiome and its role in skin barrier dysfunction and atopic dermatitis. Res. Rev. 2018. Available online: https://www.researchreview.co.nz/getmedia/48d9fa34-9f9f-4502-b8dd-c4f7b44b5d3e/Educational-Series-Skin-Microbiome-and-its-role-in-Atopic-Dermatitis-Derm.pdf.aspx?ext=.pdf (accessed on 30 June 2021).
- Skowron, K.; Bauza-kaszewska, J.; Kraszewska, Z.; Wiktorczyk-kapischke, N.; Grudlewska-buda, K.; Kwiecińska-piróg, J.; Wałecka-zacharska, E.; Radtke, L.; Gospodarek-komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.; Lee, M.J.; Norton, D.; MacLeod, A.S. The Skin and Intestinal Microbiota and Their Specific Innate Immune Systems. Front. Immunol. 2019, 10, 2950. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Tomida, S.; Nguyen, L.; Chiu, B.H.; Liu, J.; Sodergren, E.; Weinstock, G.M.; Li, H. Pan-genome and comparative genome analyses of Propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. MBio 2013, 4, e00003-13. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Du, Z.; Zhang, L.; Chen, J.; Shen, Z.; Liu, Q.; Qin, J.; Lv, H.; Wang, H.; et al. Skin microbiota analysis-inspired development of novel anti-infectives. Microbiome 2020, 8, 85. [Google Scholar] [CrossRef]
- Da Fontoura, I.C.C.; Saikawa, G.I.A.; Silveira, V.A.I.; Pan, N.C.; Amador, I.R.; Baldo, C.; da Rocha, S.P.D.; Celligoi, M.A.P.C. Antibacterial Activity of Sophorolipids from Candida bombicola Against Human Pathogens. Brazilian Arch. Biol. Technol. 2020, 63, 2020. [Google Scholar] [CrossRef]
- Lotfabad, T.B.; Shahcheraghi, F.; Shooraj, F. Assessment of Antibacterial Capability of Rhamnolipids Produced by Two Indigenous Pseudomonas aeruginosa Strains. Jundishapur J. Microbiol. 2012, 6, 29–35. [Google Scholar] [CrossRef]
- Sohn, E. Skin microbiota’s community effort. Nature 2018, 563, S91–S93. [Google Scholar] [CrossRef]
- De Freitas Ferreira, J.; Vieira, E.A.; Nitschke, M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Díaz De Rienzo, M.A.; Stevenson, P.; Marchant, R.; Banat, I.M. Antibacterial properties of biosurfactants against selected Gram-positive and -negative bacteria. FEMS Microbiol. Lett. 2016, 363, fnv224. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Navare, K.; Prabhune, A. A Biosurfactant-Sophorolipid Acts in Synergy with Antibiotics to Enhance Their Efficiency. Biomed Res. Int. 2013, 2013, 512495. [Google Scholar] [CrossRef]
- Schelges, H.; Tretyakova, M.; Ludwig, B. Cleansing Agents Containing Biosurfactants and Having Prebiotic Activity. Available online: http://www.freepatentsonline.com/y2017/0071842.html (accessed on 4 July 2020).
- Sharma, D.; Singh Saharan, B. Simultaneous production of biosurfactants and bacteriocins by probiotic lactobacillus casei MRTL3. Int. J. Microbiol. 2014, 2014, 698713. [Google Scholar] [CrossRef] [PubMed]
- Musthaq, S.; Mazuy, A.; Jakus, J. The microbiome in dermatology. Clin. Dermatol. 2018, 36, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Satpute, S.K.; Mone, N.S.; Das, P.; Banpurkar, A.G.; Banat, I.M. Lactobacillus acidophilus derived biosurfactant as a biofilm inhibitor: A promising investigation using microfluidic approach. Appl. Sci. 2018, 8, 1555. [Google Scholar] [CrossRef]
- Reid, G.; Younes, J.A.; Van der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Publ. Gr. 2011, 9, 27–38. [Google Scholar] [CrossRef]
- Rodan, K.; Fields, K.; Majewski, G.; Falla, T. Skincare Bootcamp. Plast. Reconstr. Surg.—Glob. Open 2016, 4, e1152. [Google Scholar] [CrossRef]
- Heinrich, K.; Heinrich, U.; Tronnier, H. Influence of different cosmetic formulations on the human skin barrier. Skin Pharmacol. Physiol. 2014, 27, 141–147. [Google Scholar] [CrossRef]
- Chong, H.; Li, Q. Microbial production of rhamnolipids: Opportunities, challenges and strategies. Microb. Cell Factories 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Borah, S.N.; Bora, A.; Deka, S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb. Cell Fact. 2017, 16, 95. [Google Scholar] [CrossRef]
- LaBauve, A.E.; Wargo, M.J. Growth and Laboratory Maintenance of Pseudomonas aeruginosa. Curr. Protoc. Microbiol. 2012. [Google Scholar] [CrossRef]
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. N Biotechnol. 2017, 36, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Gehring, C.; Wessel, M.; Schaffer, S.; Thum, O. The Power of Biocatalysis: A One-Pot Total Synthesis of Rhamnolipids from Butane as the Sole Carbon and Energy Source. Chemistryopen 2016, 5, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Johnravindar, D.; Wong, J.W.C.; Patria, R.D.; Kaur, G. Economical Di-Rhamnolipids Biosynthesis by Non-Pathogenic Burkholderia thailandensis E264 Using Post-Consumption Food Waste in a Biorefinery Approach. Sustainability 2023, 15, 59. [Google Scholar] [CrossRef]
- Irorere, V.U.; Smyth, T.J.; Cobice, D.; McClean, S.; Marchant, R.; Banat, I.M. Fatty acid synthesis pathway provides lipid precursors for rhamnolipid biosynthesis in Burkholderia thailandensis E264. Appl. Microbiol. Biotechnol. 2018, 102, 6163–6174. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, K.K.S.; Rahman, P.K.S.M. Rhamnolipid biosurfactants- past, present, and future scenario of global market. Front. Microbiol. 2014, 5, 454. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Wang, M.-Q.; Wang, Y.-L.; Wang, H.-T.; Xu, J.-Z.; Guo, Y.-F.; Wang, M.-Q.; Wang, Y.-L.; Wang, H.-T.; Xu, J.-Z. Controlling the Formation of Foams in Broth to Promote the Co-Production of Microbial Oil and Exopolysaccharide in Fed-Batch Fermentation. Fermentation 2022, 8, 68. [Google Scholar] [CrossRef]
- Delbeke, E.I.P.; Van Geem, K.M.; Stevens, C.V.; Van Bogaert, I.N.A. Sophorolipid Modification: The Power of Yeasts and Enzymes. In Lipid Modification by Enzymes and Engineered Microbes; AOCS Press: Ghent, Belgium, 2018; pp. 315–341. [Google Scholar]
- Ogunjobi, J.K.; McElroy, C.R.; Clark, J.H.; Thornthwaite, D.; Omoruyi, O.E.; Farmer, T.J. A class of surfactants via PEG modification of the oleate moiety of lactonic sophorolipids: Synthesis, characterisation and application. Green Chem. 2021, 23, 9906–9915. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.A.; Buyst, D.; Martins, J.C.; Roelants, S.L.K.W.; Soetaert, W.K. Synthesis of bolaform biosurfactants by an engineered Starmerella bombicola yeast. Biotechnol. Bioeng. 2016, 113, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, S.; Castelein, M.; Remmery, J.; De Clercq, V.; Lodens, S.; Baccile, N.; De Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W.K. From bumblebee to bioeconomy: Recent developments and perspectives for sophorolipid biosynthesis. Biotechnol. Adv. 2022, 54, 107788. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Munoz-Pinto, D.J.; Chen, M.; Decatur, J.; Hahn, M.; Gross, R.A. Poly(sophorolipid) structural variation: Effects on biomaterial physical and biological properties. Biomacromolecules 2014, 15, 4214–4227. [Google Scholar] [CrossRef]
- Peng, Y.; Totsingan, F.; Meier, M.A.R.; Steinmann, M.; Wurm, F.; Koh, A.; Gross, R.A. Sophorolipids: Expanding structural diversity by ring-opening cross-metathesis. Eur. J. Lipid Sci. Technol. 2015, 117, 217–228. [Google Scholar] [CrossRef]
- Van Bogaert, I.; Fleurackers, S.; Van Kerrebroeck, S.; Develter, D.; Soetaert, W. Production of new-to-nature sophorolipids by cultivating the yeast Candida bombicola on unconventional hydrophobic substrates. Biotechnol. Bioeng. 2011, 108, 734–741. [Google Scholar] [CrossRef]
- Callaghan, B.; Lydon, H.; Roelants, S.L.K.W.; Van Bogaert, I.N.A.; Marchant, R.; Banat, I.M.; Mitchell, C.A. Lactonic sophorolipids increase tumor burden in Apcmin+/− mice. PLoS ONE 2016, 11, e0156845. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Liu, Z.; Zhong, H.; Zeng, G.; Liu, G.; Yu, M.; Liu, Y.; Yang, X.; Li, Z.; Fang, Z.; et al. Effects of rhamnolipids on microorganism characteristics and applications in composting: A review. Microbiol. Res. 2017, 200, 33–44. [Google Scholar] [CrossRef]
- Li, D.; Tao, W.; Yu, D.; Li, S. Emulsifying Properties of Rhamnolipids and Their In Vitro Antifungal Activity against Plant Pathogenic Fungi. Molecules 2022, 27, 7746. [Google Scholar] [CrossRef] [PubMed]
- Radzuan, M.N.; Banat, I.M.; Winterburn, J. Production and characterization of rhamnolipid using palm oil agricultural refinery waste. Bioresour. Technol. 2017, 225, 99–105. [Google Scholar] [CrossRef]
- Tiso, T.; Zauter, R.; Tulke, H.; Leuchtle, B.; Li, W.J.; Behrens, B.; Wittgens, A.; Rosenau, F.; Hayen, H.; Blank, L.M. Designer rhamnolipids by reduction of congener diversity: Production and characterization. Microb. Cell Fact. 2017, 16, 225. [Google Scholar] [CrossRef]
- Mishra, N.; Rana, K.; Seelam, S.D.; Kumar, R.; Pandey, V.; Salimath, B.P.; Agsar, D. Characterization and Cytotoxicity of Pseudomonas Mediated Rhamnolipids Against Breast Cancer MDA-MB-231 Cell Line. Front. Bioeng. Biotechnol. 2021, 9, 761266. [Google Scholar] [CrossRef] [PubMed]
- Díaz De Rienzo, M.A.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. N Biotechnol. 2015, 32, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Semkova, S.; Antov, G.; Iliev, I.; Tsoneva, I.; Lefterov, P.; Christova, N.; Nacheva, L.; Stoineva, I.; Kabaivanova, L.; Staneva, G.; et al. Rhamnolipid Biosurfactants—Possible Natural Anticancer Agents and Autophagy Inhibitors. Separations 2021, 8, 92. [Google Scholar] [CrossRef]
- Zompra, A.A.; Chasapi, S.A.; Twigg, M.S.; Salek, K.; Anestopoulos, I.; Galanis, A.; Pappa, A.; Gutierrez, T.; Banat, I.M.; Marchant, R.; et al. Multi-method biophysical analysis in discovery, identification, and in-depth characterization of surface—Active compounds. Front. Mar. Sci. 2022, 9, 1023287. [Google Scholar] [CrossRef]
- Cosmetics Europe Guidelines for the Safety Assessment of a Cosmetic Product Guidelines for the Safety Assessment of a Cosmetic Product. Available online: https://www.cosmeticseurope.eu/files/3714/6407/8024/Guidelines_for_the_Safety_Assessment_of_a_Cosmetic_Product_-_2004.pdf (accessed on 23 December 2022).
- Commission Implementing Decision—2013/674/EU Commission Implementing Decision of 25 November 2013 on Guidelines on Annex I to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products (Text with EEA Relevance) (2013/674/EU). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013D0674&from=EN (accessed on 23 December 2022).
- Van Bogaert, I.N.A.; Zhang, J.; Soetaert, W. Microbial synthesis of sophorolipids. Process Biochem. 2011, 46, 821–833. [Google Scholar] [CrossRef]
- Phulpoto, I.A.; Yu, Z.; Hu, B.; Wang, Y.; Ndayisenga, F.; Li, J.; Liang, H.; Qazi, M.A. Production and characterization of surfactin-like biosurfactant produced by novel strain Bacillus nealsonii S2MT and its potential for oil contaminated soil remediation. Microb. Cell Fact. 2020, 19, 145. [Google Scholar] [CrossRef]
- Ciesielska, K.; Van Bogaert, I.N.; Chevineau, S.; Li, B.; Groeneboer, S.; Soetaert, W.; Van de Peer, Y.; Devreese, B. Exoproteome analysis of Starmerella bombicola results in the discovery of an esterase required for lactonization of sophorolipids. J. Proteom. 2014, 98, 159–174. [Google Scholar] [CrossRef]
- Ciesielska, K.; Roelants, S.L.K.W.; Van Bogaert, I.N.A.; De Waele, S.; Vandenberghe, I.; Groeneboer, S.; Soetaert, W.; Devreese, B. Characterization of a novel enzyme—Starmerella bombicola lactone esterase (SBLE)—Responsible for sophorolipid lactonization. Appl. Microbiol. Biotechnol. 2016, 100, 9529–9541. [Google Scholar] [CrossRef]
- Roelants, S.L.K.W.; Ciesielska, K.; De Maeseneire, S.L.; Moens, H.; Everaert, B.; Verweire, S.; Denon, Q.; Vanlerberghe, B.; Van Bogaert, I.N.A.; Van der Meeren, P.; et al. Towards the Industrialization of NewBiosurfactants: Biotechnological Opportunities forthe Lactone Esterase Gene FromStarmerellabombicola. Biotechnol. Bioeng. 2016, 113, 550–559. [Google Scholar] [CrossRef]
- Baccile, N.; Cuvier, A.S.; Valotteau, C.; Van Bogaert, I.N.A. Practical methods to reduce impurities for gram-scale amounts of acidic sophorolipid biosurfactants. Eur. J. Lipid Sci. Technol. 2013, 115, 1404–1412. [Google Scholar] [CrossRef]
- Jacobs, M.A.; Alwood, A.; Thaipisuttikul, I.; Spencer, D.; Haugen, E.; Ernst, S.; Will, O.; Kaul, R.; Raymond, C.; Levy, R.; et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2003, 100, 14339–14344. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Adu, S.A.; Sugiyama, S.; Marchant, R.; Banat, I.M. Mono-Rhamnolipid Biosurfactants Synthesized by Pseudomonas aeruginosa Detrimentally Affect Colorectal Cancer Cells. Pharmaceutics 2022, 14, 2799. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M. Isolation and Analysis of Low Molecular Weight Microbial Glycolipids. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer International Publishing: Cham, Switzerland, 2010; pp. 3705–3723. [Google Scholar]
- Inès, M.; Dhouha, G. Glycolipid biosurfactants: Potential related biomedical and biotechnological applications. Carbohydr. Res. 2015, 416, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Morita, T.; Fukuoka, T.; Imura, T.; Yanagidani, S.; Sogabe, A.; Kitamoto, D.; Kitagawa, M. The moisturizing effects of glycolipid biosurfactants, mannosylerythritol lipids, on human skin. J. Oleo Sci. 2012, 61, 407–412. [Google Scholar] [CrossRef]
- Haque, F.; Khan, M.S.A.; AlQurashi, N. ROS-Mediated Necrosis by Glycolipid Biosurfactants on Lung, Breast, and Skin Melanoma Cells. Front. Oncol. 2021, 11, 253. [Google Scholar] [CrossRef]
- Ismail, R.; Baaity, Z.; Csóka, I. Regulatory status quo and prospects for biosurfactants in pharmaceutical applications. Drug Discov. Today 2021, 26, 1929–1935. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L. Cytotoxicity testing: Measuring viable cells, dead cells, and detecting mechanism of cell death. Methods Mol. Biol. 2011, 740, 103–114. [Google Scholar] [CrossRef]
- Cummings, B.S.; Schnellmann, R.G. Measurement of Cell Death in Mammalian Cells. Curr. Protoc. 2004, 1, 1–30. [Google Scholar] [CrossRef]
- OECD Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. Available online: https://www.oecd-ilibrary.org/environment/test-no-439-in-vitro-skin-irritation-reconstructed-human-epidermis-test-method_9789264242845-en (accessed on 23 August 2022).
- Santos, D.; Rufino, R.; Luna, J.; Santos, V.; Sarubbo, L. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Shreve, G.S.; Makula, R. Characterization of a New Rhamnolipid Biosurfactant Complex from Pseudomonas Isolate DYNA270. Biomolecules 2019, 9, 885. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant Self-Assembling and Critical Micelle Concentration: One Approach Fits All? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Lotfabad, T.B.; Jabeen, F.; Mohammad Ganji, S. Cytotoxic effects of mono- and di-rhamnolipids from Pseudomonas aeruginosa MR01 on MCF-7 human breast cancer cells. Colloids Surf. B Biointerfaces 2019, 181, 943–952. [Google Scholar] [CrossRef] [PubMed]
- El-Housseiny, G.S.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. Structural and Physicochemical Characterization of Rhamnolipids produced by Pseudomonas aeruginosa P6. AMB Express 2020, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Maťátková, O.; Kolouchová, I.; Lokočová, K.; Michailidu, J.; Jaroš, P.; Kulišová, M.; Řezanka, T.; Masák, J. Rhamnolipids as a Tool for Eradication of Trichosporon cutaneum Biofilm. Biomolecules 2021, 11, 1727. [Google Scholar] [CrossRef]
- Zhang, Y.; Placek, T.L.; Jahan, R.; Alexandridis, P.; Tsianou, M. Rhamnolipid Micellization and Adsorption Properties. Int. J. Mol. Sci. 2022, 23, 11090. [Google Scholar] [CrossRef]
- Leslie, M.; Kardena, E.; Helmy, Q. Biosurfactant and Chemical Surfactant Effectiveness Test for Oil Spills Treatment in a Saline Environment; IOP Publishing Ltd: Bristol, UK, 2021. [Google Scholar]
- Juma, A.; Lemoine, P.; Simpson, A.B.J.; Murray, J.; O’Hagan, B.M.G.; Naughton, P.J.; Dooley, J.G.; Banat, I.M. Microscopic Investigation of the Combined Use of Antibiotics and Biosurfactants on Methicillin Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 1477. [Google Scholar] [CrossRef]
- Supp, D.M.; Hahn, J.M.; Combs, K.A.; McFarland, K.L.; Powell, H.M. Isolation and feeder-free primary culture of four cell types from a single human skin sample. STAR Protoc. 2022, 3, 101172. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.I.; Matin, M.M.; Bahrami, A.R.; Ghasroldasht, M.M. Immortality of cell lines: Challenges and advantages of establishment. Cell Biol. Int. 2013, 37, 1038–1045. [Google Scholar] [CrossRef]
- Teimouri, A.; Yeung, P.; Agu, R.; Teimouri, A.; Yeung, P.; Agu, R. 2D vs. 3D Cell Culture Models for In Vitro Topical (Dermatological) Medication Testing. In Cell Culture; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910. [Google Scholar] [CrossRef]
- Park, Y.; Huh, K.M.; Kang, S.W. Applications of Biomaterials in 3D Cell Culture and Contributions of 3D Cell Culture to Drug Development and Basic Biomedical Research. Int. J. Mol. Sci. 2021, 22, 2491. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.B.; Bojar, R.A.; Farrar, M.D.; Holland, K.T. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol. Lett. 2009, 290, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Hosseini, M.; Vainio, S.; Taïeb, A.; Cario-André, M.; Rezvani, H.R. Skin equivalents: Skin from reconstructions as models to study skin development and diseases. Br. J. Dermatol. 2015, 173, 391–403. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Donaldson, M.; Francese, S.; Clench, M.R. MALDI MSI analysis of lipid changes in living skin equivalents in response to emollient creams containing palmitoylethanolamide. Methods 2016, 104, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.J.; Tamburic, S. A State-of-the-Art Review on the Alternatives to Animal Testing for the Safety Assessment of Cosmetics. Cosmetics 2022, 9, 90. [Google Scholar] [CrossRef]
- Duffy, E.; De Guzman, K.; Wallace, R.; Murphy, R.; Morrin, A. Non-Invasive Assessment of Skin Barrier Properties: Investigating Emerging Tools for In Vitro and In Vivo Applications. Cosmetics 2017, 4, 44. [Google Scholar] [CrossRef]
- Ma, X.; Wang, F.; Wang, B. Application of an in vitro reconstructed human skin on cosmetics in skin irritation tests. J. Cosmet. Dermatol. 2021, 20, 1933–1941. [Google Scholar] [CrossRef]
- De Wever, B.; Petersohn, D.; Mewes, K.R. Overview of human three-dimensional (3D) skin models used for dermal toxicity assessment. Househ. Pers. Care Today 2013, 8, 18–23. [Google Scholar]
- Klicks, J.; von Molitor, E.; Ertongur-Fauth, T.; Rudolf, R.; Hafner, M. In vitro skin three-dimensional models and their applications. J. Cell. Biotechnol. 2017, 3, 21–39. [Google Scholar] [CrossRef]
- Bojar, R.A. Studying the Human Skin Microbiome Using 3D In Vitro Skin Models. Appl. Vitr. Toxicol. 2015, 1, 165–171. [Google Scholar] [CrossRef]
- Larson, P.; Chong, D.; Fleming, E.; Oh, J. Challenges Developing a Human Model System for Skin Microbiome Research HHS Public Access. J. Investig. Dermatol. 2021, 141, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.E.L.; Barrett, M.R.T.; Freeman-Parry, L.; Bojar, R.A.; Clench, M.R. Examination of the skin barrier repair/wound healing process using a living skin equivalent model and matrix-assisted laser desorption-ionization-mass spectrometry imaging. Int. J. Cosmet. Sci. 2018, 40, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ohadi, M.; Forootanfar, H.; Dehghannoudeh, N.; Banat, I.M.; Dehghannoudeh, G. The role of surfactants and biosurfactants in the wound healing process: A review. J. Wound Care 2023, 32, xxxix-xlvi. [Google Scholar] [CrossRef] [PubMed]



| Glycolipid Subclass | Effect | References |
|---|---|---|
| All glycolipids | Affect surface chemistry of the skin, promoting skin commensal bacteria | [85,86,87,88] |
| Provide antibiotic synergy against Gram-negative pathogens | [54,94,95] | |
| Rhamnolipids | Antimicrobial effects on Gram-positive bacteria | [93] |
| No detrimental effects on human HaCaT keratinocyte cell line | [33,38] | |
| Detrimentally affect SK-Mel-28 melanoma cell line | [33] | |
| Sophorolipids | Antimicrobial effect on Staphylococcus aureus | [92] |
| Antimicrobial effect on Streptococcus pyogenes | [92] | |
| Antimicrobial effect on Cutibacterium acnes | [92] | |
| Accelerate dermal wound healing in vitro | [27] | |
| Stimulate Col-1 gene expression | [27] | |
| Inhibit elastase enzymes | [27] | |
| No detrimental effects on different healthy human skin cell lines | [27,33,59] | |
| Attenuate gene expression of proinflammatory cytokines | [27] | |
| Detrimentally affect SK-Mel-28 melanoma cell line | [33] | |
| Uncharacterized glycolipids | Antimicrobial effect on Staphylococcus aureus | [97] |
| Antimicrobial effect on Staphylococcus epidermidis | [97] | |
| Antimicrobial effect on Pseudomonas aeruginosa | [97] | |
| Antimicrobial effect on Salmonella typhi | [97] | |
| Antimicrobial effect on Escherichia coli | [100] | |
| Antimicrobial effect on Bacillus subtilis | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Glycolipid Biosurfactants in Skincare Applications: Challenges and Recommendations for Future Exploitation. Molecules 2023, 28, 4463. https://doi.org/10.3390/molecules28114463
Adu SA, Twigg MS, Naughton PJ, Marchant R, Banat IM. Glycolipid Biosurfactants in Skincare Applications: Challenges and Recommendations for Future Exploitation. Molecules. 2023; 28(11):4463. https://doi.org/10.3390/molecules28114463
Chicago/Turabian StyleAdu, Simms A., Matthew S. Twigg, Patrick J. Naughton, Roger Marchant, and Ibrahim M. Banat. 2023. "Glycolipid Biosurfactants in Skincare Applications: Challenges and Recommendations for Future Exploitation" Molecules 28, no. 11: 4463. https://doi.org/10.3390/molecules28114463
APA StyleAdu, S. A., Twigg, M. S., Naughton, P. J., Marchant, R., & Banat, I. M. (2023). Glycolipid Biosurfactants in Skincare Applications: Challenges and Recommendations for Future Exploitation. Molecules, 28(11), 4463. https://doi.org/10.3390/molecules28114463