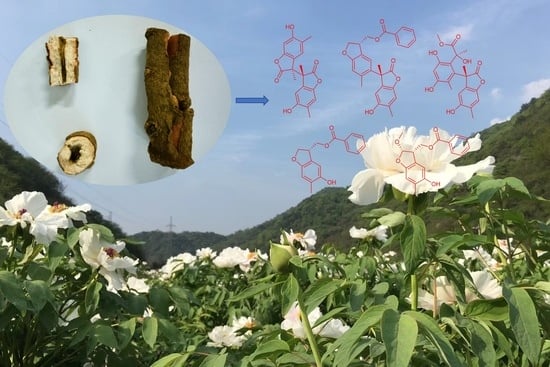
New Phenolic Dimers from Plant Paeonia suffruticosa and Their Cytotoxicity and NO Production Inhibition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of Compounds 1–5
2.2. Bioactivity Analysis
3. Experiments
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Paeobenzofuranone A (1)
3.3.2. Paeobenzofuranone B (2)
3.3.3. Paeobenzofuranone C (3)
3.3.4. Paeobenzofuranone D (4)
3.3.5. Paeobenzofuranone E (5)
3.4. Cytotoxicity Assay
3.5. NO Inhibitory Activity Assays
3.6. ECD Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, L.; Zhao, F.; Chen, L.; Jiang, Z.; Liu, Y.; Li, Z.; Qiu, F.; Yao, X. New monoterpene glycosides from Paeonia suffruticosa Andrews and their inhibition on NO production in LPS-induced RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2012, 22, 7243–7247. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Tang, S.W.; Lam, S.H.; Wang, C.C.; Liu, Y.H.; Lin, H.Y.; Lee, S.S.; Lin, J.Y. Aqueous extract of Paeonia suffruticosa inhibits migration and metastasis of renal cell carcinoma cells via suppressing VEGFR-3 pathway. Evid. Based Compl. Alt. 2012, 2012, 409823. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Zhang, L.; Zhu, M.; Zhang, M.; Chen, J.; Feng, L.; Jia, X.; Jacob, J.A. Capture of anti-coagulant active ingredients from Moutan Cortex by platelet immobilized chromatography and evaluation of anticoagulant activity in rats. Biomed. Pharmacother. 2017, 95, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Jie, S.; Chunnian, H.; Pei, X. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef]
- Mencherini, T.; Picerno, P.; Festa, M.; Russo, P.; Capasso, A.; Aquino, R. Triterpenoid constituents from the roots of Paeonia rockii ssp. rockii. J. Nat. Prod. 2011, 74, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ohno, O.; Suenaga, K.; Miyamoto, K. Apoptosis-inducing activity and antiproliferative effect of paeoniflorigenone from Moutan Cortex. Biosci. Biotechnol. Biochem. 2017, 81, 1106–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Wang, Y.; Gao, J.; Lu, Z.; Yin, W.; Deng, R. Resveratrol trimers from seed cake of Paeonia rockii. Molecules 2014, 19, 19549–19556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuya, R.; Hu, H.; Zhang, Z.; Shigemori, H. Suffruyabiosides A and B, two new monoterpene diglycosides from Moutan Cortex. Molecules 2012, 17, 4915–4923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.K.; Ma, Y.B.; Wu, D.G.; Lu, Y.; Shen, Z.Q.; Zheng, Q.T.; Chen, Z.H. Paeonilide, a novel anti-PAF active monoterpenoid derived metabolite from Paeonia delavayi. Biosci. Biotech. Biochem. 2000, 64, 1511–1514. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.Y.; Feng, T.; Li, Z.H.; Sheng, Y.; Yin, X.; Leng, Y.; Liu, J.K. Conosilane A, an unprecedented sesquiterpene from the Cultures of Basidiomycete Conocybe siliginea. Org. Lett. 2012, 14, 5382–5384. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Y.; Zhang, L.; Liu, J.K. Bi-linderone, a highly modified methyl-linderone dimer from Lindera aggregata with activity toward improvement of insulin sensitivity in vitro. Org. Lett. 2010, 12, 2354–2357. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Hsieh, K.L.; Liu, J.K. Guajadial: An unusual meroterpenoid from guava leaves Psidium guajava. Org. Lett. 2007, 9, 5135–5138. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Su, J.; Ding, Z.H.; Zheng, Y.T.; Li, Y.; Leng, Y.; Liu, J.K. Chemical constituents and their bioactivities of Tongling White Ginger (Zingiber officinale). J. Agric. Food Chem. 2011, 59, 11690–11695. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.G.; Cheng, C.Q.; Liu, J.K. X-ray Crystal Structure of Angulatusine A, a new sesquiterpene alkaloid from Celastrus Angulatus. J. Nat. Prod. 1992, 55, 982–985. [Google Scholar] [CrossRef]
- Ha, D.T.; Ngoc, T.M.; Lee, I.S.; Lee, Y.M.; Kim, J.S.; Jung, H.J. Inhibitors of aldose reductase and formation of advanced glycation end-products in moutan cortex (Paeonia suffruticosa). J. Nat. Prod. 2009, 72, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Long, Q.; Yi, W.F.; Yang, B.J.; Ding, X.; Hao, X.J. Two new C21 steroidal glycosides from the roots of Cynanchum paniculatum. Nat. Prod. Bioprospect. 2019, 4, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Anh, H.L.T.; Cuc, N.T.; Tai, B.H.; Yen, P.H.; Nhiem, N.X.; Thao, D.T.; Nam, N.H.; Minh, C.V.; Kiem, P.V.; Kim, Y.H. Synthesis of chromonylthiazolidines and their cytotoxicity to human cancer cell Lines. Molecules 2015, 20, 1151–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snene, A.; Mokni, R.E.; Jmii, H.; Jlassi, I.; Jadane, H.; Falconieri, D.; Piras, A.; Dhaouadi, H.; Porcedda, S.; Hammami, S. In vitro antimicrobial, antioxidant and antiviral activities of the essential oil and various extracts of wild (Daucus virgatus (Poir.) Maire) from Tunisia. Ind. Crops Prod. 2017, 109, 109–115. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumloeffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]




| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | 4.46, m | 5.48, t (1.8) | |||
| 3 | 3.81, t (7.4) | 3.80, t (7.5) | |||
| 4 | 7.18, s | 6.61, s | 6.96, s | 6.74, s | 6.72, s |
| 7 | 6.61, s | 6.52, s | 6.33, s | 4.44, dd (10.7, 5.9) 4.37, dd (18.0, 9.1) | 4.46, dd (11.1, 5.5) 4.33, dd (11.1, 7.8) |
| 10 | 1.67, s, 3H | 1.75, s, 3H | 1.72, s, 3H | 4.43, m | 4.48, m |
| 11 | 2.15, s, 3H | 2.13, s, 3H | 2.02, s, 3H | 2.14, s, 3H | 2.15, s, 3H |
| 12 | 3.48, s | ||||
| 2′ | 4.43, m; 4.06, m | 8.00, d (1.5) | 7.97, d (1.5) | ||
| 3′ | 3.81, t (7.5) | 7.44, m | 7.45, m | ||
| 4′ | 7.18, s | 6.63, s | 6.88, s | 7.58, m | 7.61, m |
| 5′ | 6.61, s | 6.62, s | 7.49, m | 7.48, m | |
| 6′ | 1.67, s, 3H | 8.01, d (1.3) | 7.99, d (1.3) | ||
| 7′ | 2.15, s, 3H | 6.85, s | |||
| 9′ | 3.73, s | ||||
| 10′ | 4.60, dd (18.0, 9.1) 4.44, dd (10.8, 5.9) | 1.63, s, 3H | |||
| 11′ | 2.05, s, 3H | 1.77, s, 3H | |||
| 3′′ | 8.01, d (1.2) | ||||
| 4′′ | 7.48, t (7.8) | ||||
| 5′′ | 7.56, m | ||||
| 6′′ | 7.48, t (7.8) | ||||
| 7′′ | 8.00, d (1.4) |
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | 179.4, C | 179.3, C | 182.7, C | 73.5, CH2 | 111.2, CH |
| 3 | 52.6, C | 52.6, C | 50.2, C | 42.0, CH | 50.5, CH |
| 4 | 128.1, CH | 112.2, CH | 115.6, CH | 110.8, CH | 112.6, CH |
| 5 | 113.3, C | 149.3, C | 149.2, C | 148.9, C | 150.9, C |
| 6 | 153.7, C | 126.3, C | 125.0, C | 128.5, CH | 124.4, CH |
| 7 | 110.3, CH | 112.4, CH | 118.9, CH | 110.6, CH | 112.3, CH |
| 8 | 146.2, C | 125.9, C | 144.3, C | 153.4, C | 153.2, C |
| 9 | 127.4, C | 127.4, C | 126.4, C | 125.6, C | 129.8, C |
| 10 | 18.7, CH3 | 18.6, CH3 | 22.3, CH3 | 66.4, CH2 | 66.3, CH2 |
| 11 | 16.6, CH3 | 16.6, CH3 | 15.9, CH3 | 15.6, CH3 | 17.0, CH3 |
| 12 | 56.2, OCH3 | ||||
| 1′ | 133.7, C | ||||
| 2′ | 179.4, C | 73.1, CH2 | 153.2, C | ||
| 3′ | 52.6, C | 43.5, CH | 122.0, C | ||
| 4′ | 128.1, CH | 111.7, CH | 115.9, CH | ||
| 5′ | 113.3, C | 126.4, C | 144.6, C | ||
| 6′ | 153.7, C | 131.4, CH | 125.7, C | ||
| 7′ | 110.3, CH | 110.3, CH | 75.1, C | ||
| 8′ | 146.2, C | 154.9, C | 176.8, C | ||
| 9′ | 127.4, C | 124.4, C | 53.2, OCH3 | ||
| 10′ | 18.7, CH3 | 66.9, CH2 | 26.0, CH3 | ||
| 11′ | 16.6, CH3 | 16.8, CH3 | 10.4, CH3 | ||
| 1′′ | 168.1, C | 166.5, C | 167.9, C | ||
| 2′′ | 130.7, C | 129.7, C | 131.2, C | ||
| 3′′ | 129.7, CH | 129.2, CH | 130.4, CH | ||
| 4′′ | 128.1, CH | 128.2, CH | 129.5, CH | ||
| 5′′ | 134.5, CH | 132.9, CH | 130.7, CH | ||
| 6′′ | 129.7, CH | 128.2, CH | 129.5, CH | ||
| 7′′ | 128.1, CH | 129.1, CH | 130.4, CH |
| Compound | Inhibition Activity (100%) |
|---|---|
| L-NMMA a | 52.0 ± 1.96 |
| 1 | 43.9 ± 2.07 |
| 2 | 44.6 ± 0.52 |
| 3 | 13.0 ± 1.59 |
| 4 | 33.7 ± 2.24 |
| 5 | 30.9 ± 1.56 |
| Compound | HL-60 | MDA-MB-231 | SW480 |
|---|---|---|---|
| 2 | 6.8 ± 0.11 | 20.9 ± 0.46 | 12.6 ± 0.73 |
| 4 | 19.1 ± 0.32 | >40 | 8.9 ± 0.40 |
| 5 | 11.1 ± 1.61 | >40 | 10.7 ± 0.43 |
| DDP a | 23.5 ± 0.77 | 16.9 ± 1.19 | 25.1 ± 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Tong, S.; Zhao, Y.; Peng, X.; Li, Z.; Feng, T.; Liu, J. New Phenolic Dimers from Plant Paeonia suffruticosa and Their Cytotoxicity and NO Production Inhibition. Molecules 2023, 28, 4590. https://doi.org/10.3390/molecules28124590
Meng Q, Tong S, Zhao Y, Peng X, Li Z, Feng T, Liu J. New Phenolic Dimers from Plant Paeonia suffruticosa and Their Cytotoxicity and NO Production Inhibition. Molecules. 2023; 28(12):4590. https://doi.org/10.3390/molecules28124590
Chicago/Turabian StyleMeng, Qianqian, Shunyao Tong, Yuqing Zhao, Xingrong Peng, Zhenghui Li, Tao Feng, and Jikai Liu. 2023. "New Phenolic Dimers from Plant Paeonia suffruticosa and Their Cytotoxicity and NO Production Inhibition" Molecules 28, no. 12: 4590. https://doi.org/10.3390/molecules28124590
APA StyleMeng, Q., Tong, S., Zhao, Y., Peng, X., Li, Z., Feng, T., & Liu, J. (2023). New Phenolic Dimers from Plant Paeonia suffruticosa and Their Cytotoxicity and NO Production Inhibition. Molecules, 28(12), 4590. https://doi.org/10.3390/molecules28124590