Mutual Solubilities between Ethylene Glycol and Organic Diluents: Gas Chromatography and NMR
Abstract
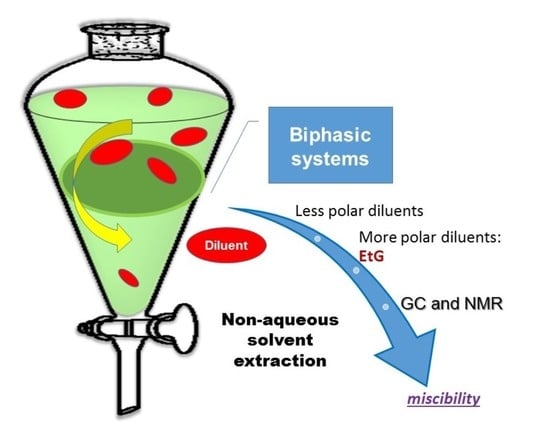
:1. Introduction
2. Results and Discussion
2.1. Studies Based on Gas Chromatography
2.2. NMR Investigation
3. Materials and Methods
3.1. Materials
3.2. Analytical Methodology
3.3. Kinetic Solvent Extraction Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Janssen, C.; Marcias-Ruvalcaba, N.; Aguilar-Martinez, M.; Kobrak, M. Metal extraction to ionic liquids: The relationship between structure, mechanism and application. Int. Revs. Phys. Chem. 2015, 34, 591–622. [Google Scholar] [CrossRef]
- Okamura, H.; Aoyagi, N.; Shimojo, K.; Naganawa, H.; Imura, H. Role of Tf2N- anions in the ionic liquid-water distribution of europium(III) chelates. RSC Adv. 2017, 7, 7610–7618. [Google Scholar] [CrossRef] [Green Version]
- Turanov, A.N.; Karandashev, V.K.; Baulin, D.V.; Baulin, V.E.; Tsivadze, A.Y. Solvent extraction of lanthanides(III) with mixtures of 1,3,5-tris(2-diphenylphosphoryl-4-ethylphenoxymethyl)benzene and 4-benzoyl-5-methyl-2-phenyl-3-pyrazolone. Russ. Chem. Bull. 2021, 70, 2416. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Liu, Y.; Wang, L.; Wu, F.; Wang, X. A novel green diluent for the preparation of poly(4-methyl-1-pentene) membranes via a thermally-induced phase separation method. Membranes 2021, 11, 622. [Google Scholar] [CrossRef]
- Petrova, M. The Crucial Performance of Mutual Solubility among Water and Ionic Liquids in the Time of Liquid-Liquid Extraction of Metallic Species; Academic Solutions: Sofia, Bulgaria, 2020; pp. 1–159. ISBN 978-954-2940-23-4. [Google Scholar]
- Atanassova, M. Solvent extraction chemistry in ionic liquids: An overview of f-ions. J. Mol. Liq. 2021, 343, 117530. [Google Scholar] [CrossRef]
- Atanassova, M. Solvent extraction of metallic species in ionic liquids: An overview of s-, p- and d-elements. J. Chem. Technol. Met. 2021, 3, 443. [Google Scholar]
- Qiu, L.; Li, J.; Zhang, W.; Gaong, A.; Yuan, X.; Liu, Y. Extraction and back-extraction behaviours of La(III), Ce(III), Pr(III), and Nd(III) single rare earth and mixed rare earth by TODGA. Sensors 2021, 21, 8316. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. High Molecular Weight Amines and Quaternary Ammonium Salts as Synergistic Agents in the Solvent Extraction of Metal Ions with Chelating Extractants. Chap. 7. In Handbook of Inorganic Chemistry Researc; Morrison, D.A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 245–267. [Google Scholar]
- Atanassova, M. Assessment of the equilibrium constants of mixed complexes of rare earth elements with acidic (chelating) and organophosphorus ligands. Separations 2022, 9, 371. [Google Scholar] [CrossRef]
- Marcus, Y. Diluent effects in solvent extraction. Solvent Extr. Ion Exch. 1989, 7, 567. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. Effect of the diluents on the synergistic solvent extraction of some lanthanides with thenoyltrifluoroacetone and quaternary ammonium salt. Hydrometallurgy 2003, 68, 89–96. [Google Scholar] [CrossRef]
- Petrova, M.; Lachkova, V.; Vassilev, G.; Varbanov, S. Effect of the diluents on the synergistic solvent extraction and separation of trivalent lanthanoids with 4-benzoyl-3-phenyl-5-isoxazolone and tert-butylcalix[4]arene tetrakis(N,N-dimethyl acetamide) and structural study of Gd(III) solid complex by IR and NMR. Ind. Eng. Chem. Res. 2010, 49, 6189–6195. [Google Scholar]
- Gupta, K.; Achuthan, P.; Ramanujam, A.; Mathur, J. Effect of diluents on the extraction of Sr2+ from HNO3 solutions with dicyclohexano-18-crown-6. Solvent Extr. Ion Exch. 2003, 21, 53–71. [Google Scholar] [CrossRef]
- El-Hefny, N.; El-Nadi, Y.; Daoud, J. Effect of diluents on the extraction of zirconium from nitrate medium by thenoyltrifluoroacetone. Solvent Extr. Ion Exch. 2006, 24, 703–717. [Google Scholar] [CrossRef]
- Turanov, A.; Karandashev, V.; Artyushin, O.; Sharova, E. Extraction of U(VI), Th(IV), and lanthanids(III) from nitric acid solutions with CMPO-functionalized ionic liquid in molecular diluents. Solvent Extr. Ion Exch. 2015, 33, 540–553. [Google Scholar] [CrossRef]
- He, J.; Tao, W.; Dong, G. Study on extraction performance of vanadium(V) from aqueous solutions by octyl-imidazole ionic liquids extractants. Metals 2022, 12, 854. [Google Scholar] [CrossRef]
- Atanassova, M. Thenoyltrifluoroacetone: Preferable molecule for solvent extraction of metals—Ancient twists to new approaches. Separations 2022, 9, 154. [Google Scholar] [CrossRef]
- Kirchnerova, J.; Cave, G. The solubility of water in low-dielectric solvents. Can. J. Chem. 1976, 54, 3909–3916. [Google Scholar] [CrossRef] [Green Version]
- Winkelman, J.; Kraai, G.; Heeres, H. Binary, ternary and quternay liquid-liquid equilibria in 1-butanol, oleic acid, water and n-heptane mixtures. Fluid Phase Equilib. 2009, 284, 71–79. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P. Solvometallurgy: An emerging branch of extractive metallurgy. J. Sustain. Metall. 2017, 3, 570. [Google Scholar] [CrossRef] [Green Version]
- Wellens, S.; Thijs, B.; Möller, C.; Binnemans, K. Separation of cobalt and nickel by solvent extraction with two mutually immiscible ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 9663–9669. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Dewulf, B.; Binnemans, K. Nonaqueous solvent extraction for enhanced metal separations: Concept, systems, and mechanisms. Ind. Eng. Chem. Res. 2021, 60, 17285. [Google Scholar] [CrossRef] [PubMed]
- Batchu, N.K.; Dewulf, B.; Riano, S.; Binnemans, K. Development of a solvometallurgical process for the separation of yttrium and europium by Cyanex 923 from ethylene glycol solutions. Sep. Purif. Technol. 2020, 235, 116193. [Google Scholar] [CrossRef]
- Dewulf, B.; Riano, S.; Binnemans, K. Separation of heavy rare-earth elements by non-aqueous solvent extraction: Flowsheet development and mixer-settler tests. Sep. Purif. Technol. 2022, 290, 1220882. [Google Scholar] [CrossRef]
- Rizk, S.; Gamal, R.; El-Hefny, N. Insights into non-aqueous solvent extraction of gadolinium and neodymium from ethylene glycol solution using Cyanex 572. Sep. Purif. Technol. 2021, 275, 119160. [Google Scholar] [CrossRef]
- Atanassova, M.; Kukeva, R. Improvement of Gd(III) solvent extraction by 4-benzoyl-3-methyl-1-phenyl-2-pyazolin-5-one: Non aqueous systems. Separations 2023, 10, 286. [Google Scholar] [CrossRef]
- Rickert, P.; Stepinski, D.; Rausch, D.; Bergeron, R.; Jakab, S.; Dietz, M. Solute-induced dissolution of hydrophobic ionic liquids in water. Talanta 2007, 72, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Goes, M.; Franceschi, E.; Santos, A.; Dariva, C.; Fortuny, M.; Mattedi, S. Phase equilibria for binary systems containing ionic liquid with water or hydrocarbons. Braz. J. Chem. Eng. 2015, 32, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Ranke, J.; Othman, A.; Fan, P.; Müller, A. Explaining ionic liquid water solubility in terms of cation and anion hydrophobicity. Int. J. Mol. Sci. 2009, 10, 1271–1289. [Google Scholar] [CrossRef]
- Malchanova, K.; Jacquemin, J.; Wagner, Z.; Bendova, M. Mutual solubilities of ammonium-based ionic liquids with water and with wather/ethanol mixture. Procedia Eng. 2012, 42, 1229–1241. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, K.; Haider, N.; Yusuf, M.; Hussain, A.; Afzal, O.; Yasmin, S.; Altamimie, A. Water/transcutol/lecithin/M−812 green cationic nanoemulsion to treat oxytetracycline contaminated aqueous bulk solution. J. Mol. Liq. 2022, 357, 119154. [Google Scholar] [CrossRef]
- Afzal, O.; Alshammari, H.; Altamimi, M.; Hussain, A.; Almohaywi, B.; Altamimi, A. Hansen solubility parameters and green nanocarrier based removal of trimethoprim from contaminated aqueous solution. J. Mol. Liq. 2022, 361, 119657. [Google Scholar] [CrossRef]
- Atanassova, M.; Mazan, V.; Billard, I. Modulating the solubilities of ionic liquid components in aqueous-ionic liquid biphasic systems: A Q-NMR investigation. ChemPhysChem 2015, 16, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, M.; Kurteva, V. Peculiar synergistic extraction behavior of Eu(III) in ionic liquids: Benzoylacetone and CMPO fusion. Sep. Purif. Technol. 2017, 183, 226–236. [Google Scholar] [CrossRef]
- Mazan, V.; Billard, I.; Papaiconomou, N. Experimental connections between aqueous aqueous and aqueous ionic biphasic systems. RSC Adv. 2014, 4, 13371–13384. [Google Scholar] [CrossRef]
- Atanassova, M.; Billard, I. Determination of pKaIL values of three chelating extractants in ILs. Consequences on extraction process of 4f-elements. J. Solut. Chem. 2015, 44, 606–620. [Google Scholar] [CrossRef]
- Atanasova, M.; Kurteva, V. Synergism in the solvent extraction of europium(III) with thenoyltrifluoroacetone and CMPO in methylimidazolium ionic liquids. J. Solut. Chem. 2019, 48, 15–30. [Google Scholar] [CrossRef]
- Ternova, D.; Boltoeva, M.; Cointeaux, L.; Gaillard, C.; Kalchenko, V.; Mazan, V.; Miroshnichenko, S.; Mohapatra, P.; Ouadi, A.; Papaiconomou, N.; et al. Dramatic changes in the solubilities of ions induced by ligand addition in biphasic system D2O/DNO3/[C1C4im][Tf2N]: A phenomenological study. J. Phys. Chem. B. 2016, 120, 7502–7510. [Google Scholar] [CrossRef]
- Inoue, S.; Zhang, Q.; Uto, M. Distribution equilibrium of lanthanide (III) complexes with N-benzoyl-N-phenylhydroxylamine in several inert solvent systems. Solvent Extr. Ion Exch. 2000, 18, 441–450. [Google Scholar] [CrossRef]
- Micchieraldo, R.; Gehrke, S.; Batchu, N.; Kirchner, B.; Binnemans, K. Tuning solvent miscibility: Fundamental assessment of the example of induced methanol/n-dodecan phase separation. J. Phys. Chem. B 2019, 123, 4400–4407. [Google Scholar] [CrossRef]
- Kolarik, Z. The effect of diluent on the solvent extraction of Tb(III) and Eu(III) by di-n-octyl phosphoric acid. In Solvent Extraction Chemistry; Dyrssen, D., Liljenzin, J.-O., Rydberg, J., Eds.; John Wiley: Amsterdam, The Netherlands, 1967; pp. 250–255. [Google Scholar]
- Batchu, N.K.; Vander Hoogerstrete, T.; Banerjee, D.; Binnemans, K. Non-aqueous solvent extraction of rare-earth nitrates from ethylene glycol to n-dodecane by Cyanex 923. Sep. Purif. Technol. 2017, 174, 544–553. [Google Scholar] [CrossRef]
- Batchu, N.K.; Vander Hoogerstraete, T.; Banerjee, D.; Binnemans, K. Separation of rare-earth ions from ethylene glycol (+LiCl) solution by non-aqueous solvent extraction with Cyanex 923. RSC Adv. 2017, 7, 45351–45362. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.; d’Acampora Zellner, B.; Crupi, M.L.; de Fina, M.R.; Valentino, M.R.; Dugo, P.; Dugo, G.; Mondello, L. GC–MS, GC–O and enantio–GC investigation of the essential oil of Tarchonanthus camphoratus L. Flavour Fragr. J. 2008, 23, 40–48. [Google Scholar] [CrossRef]
- Kretzschmar, N.; Seifert, M.; Busse, O.; Weigand, J. Prediction of retention indices and response factors of oxygenates for GC-FID by multilinear regression. Data 2022, 7, 133. [Google Scholar] [CrossRef]





| Sample | Ethylene Glycol | Organic Diluent | ||
|---|---|---|---|---|
| EtG ↔ C6H6 | 96.1% (61.8%) | 3.9% (38.2%) | 99.9% (96.2%) | 0.1% (3.8%) |
| EtG ↔ CHCl3 | 75.5% (80.1%) | 24.5% (17.1%) | 98.4% (88.2%) | 1.6% (8.9%) |
| EtG ↔ C2H4Cl2 | 90.1% (89.1%) | 9.9% (10.9%) | 99.5% (97.1%) | 0.5% (2.4%) |
| Sample | 1,2-/1,3-Propandiol | Organic Diluent | ||
|---|---|---|---|---|
| 1,2-propandiol ↔ C6H12 | 97.2% | 2.8% | 100% | 0% |
| 1,3-propandiol ↔ C6H12 | 98.8% | 1.2% | 100% | 0% |
| 1,3-propandiol ↔ CHCl3 | 72.3% | 27.7% | 98.8% | 1.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanassova, M.; Kurteva, V. Mutual Solubilities between Ethylene Glycol and Organic Diluents: Gas Chromatography and NMR. Molecules 2023, 28, 5121. https://doi.org/10.3390/molecules28135121
Atanassova M, Kurteva V. Mutual Solubilities between Ethylene Glycol and Organic Diluents: Gas Chromatography and NMR. Molecules. 2023; 28(13):5121. https://doi.org/10.3390/molecules28135121
Chicago/Turabian StyleAtanassova, Maria, and Vanya Kurteva. 2023. "Mutual Solubilities between Ethylene Glycol and Organic Diluents: Gas Chromatography and NMR" Molecules 28, no. 13: 5121. https://doi.org/10.3390/molecules28135121
APA StyleAtanassova, M., & Kurteva, V. (2023). Mutual Solubilities between Ethylene Glycol and Organic Diluents: Gas Chromatography and NMR. Molecules, 28(13), 5121. https://doi.org/10.3390/molecules28135121