Synthesis and Properties of New 3-Heterylamino-Substituted 9-Nitrobenzanthrone Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. NMR Spectra Analysis
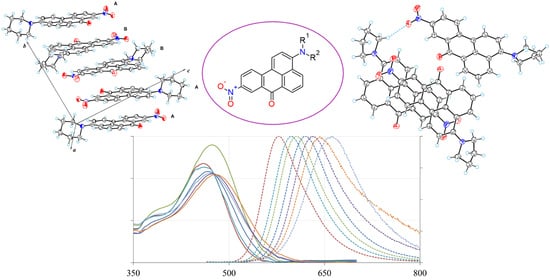
2.3. X-ray Crystallographic Study
2.4. Spectroscopic Properties
3. Materials and Methods
3.1. Materials and Basic Measurements
3.2. Synthesis and Characterization
- 3-Morpholino-9-nitro-7H-benzo[de]anthracen-7-one (2), Obtained as a red compound in a 58% yield with an m.p. of 229–230 °C. Rf = 0.63 (eluent C6H6-CH3CN, v/v 3:1). 1H NMR (500 MHz, CDCl3) δ 9.19 (d, J = 2.4 Hz, 1H, (8)), 8.75 (d, J = 7.3 Hz, 1H, (6)), 8.55 (d, J = 8.3 Hz, 1H, (4)), 8.39 (d, J = 6.8 Hz, 1H, (10)), 8.38 (d, J = 6.5 Hz, 1H, (1)), 8.30 (d, J = 8.9 Hz, 1H, (11)), 7.76 (t, J = 7.8 Hz, 1H, (5)), 7.21 (d, J = 8.1 Hz, 1H, (2)), 3.98 (t, J = 4.5 Hz, 4H, (2′, 6′)), 3.21 (t, J = 4.5 Hz, 4H, (3′, 5′)). 13C NMR (126 MHz, CDCl3) δ 182.17 (C=O), 154.28 (C), 146.61 (C), 141.30 (C), 131.65 (CH, (4)), 130.86 (CH, (6)), 130.46 (C), 129.76 (C), 128.57 (C), 127.88 (C), 127.46 (CH, (1)), 126.82 (CH, (10)), 126.29 (CH, (5), 123.94 (CH, (11)), 123.72 (CH, (8)), 119.77 (C), 115.10 (CH, (2)), 67.08 (CH2, (2′, 6′)), 53.79 (CH2, (3′, 5′)). FTIR (neat): 655, 649, 709, 744, 756, 768, 794, 804, 833, 872, 903, 925, 945, 954, 979, 1024, 1040, 1052, 1067, 1081, 1092, 1126, 1157, 1179, 1212, 1249, 1278, 1303, 1319, 1361, 1385, 1407, 1439, 1460, 1477, 1506, 1569, 1582, 1595, 1646, 2885, 2991, 3054. ESI-FTMS: calculated for [C21H16N2O4 + H+]: 361.1183, found: 361.1181.
- 9-Nitro-3-(pyrrolidin-1-yl)-7H-benzo[de]anthracen-7-one (3), Obtained as a red compound in a 63% yield with an m.p. of 257–258 °C. Rf = 0.73 (eluent C6H6-CH3CN, v/v 3:1). 1H NMR (500 MHz, CDCl3) δ 9.21 (d, J = 2.6 Hz, 1H, (8)), 8.78 (d, J = 7.3 Hz, 1H, (6)), 8.65 (d, J = 8.4 Hz, 1H, (4)), 8.33 (dd, J = 8.9, 2.6 Hz, 1H, (10)), 8.26 (d, J = 8.7 Hz, 1H, (1)), 8.20 (d, J = 8.9 Hz, 1H, (11)), 7.63 (dd, J = 7.9 Hz, 1H, (5)), 6.83 (d, J = 8.7 Hz, 1H, (2)), 3.76–3.70 (m, 4H, (2′, 5′)), 2.09–2.03 (m, 4H, (3′, 4′)). 13C NMR (126 MHz, CDCl3) δ 133.24 (CH, (4)), 130.99 (CH, (6)), 128.92 (CH, (1)), 126.42 (CH, (10)), 124.07 (CH, (8)), 123.72 (CH, (5)), 123.11 (CH, (11)), 108.86 (CH, (2)), 53.48 (CH2, (2′, 5′)), 26.10 (CH2, (3′, 4′)). FTIR (neat): 661, 675, 696, 743, 761, 768, 796, 818, 841, 861, 875, 890, 921, 966, 1007, 1039, 1072, 1100, 1113, 1146, 1169, 1213, 1242, 1266, 1290, 1312, 1344, 1405, 1444, 1497, 1524, 1556, 1567, 1582, 1603, 1644, 2847, 2959, 3083. ESI-FTMS: calculated for [C21H16N2O3 + H+]: 345.1234, found: 345.1232.
- 3-(4-Methylpiperazin-1-yl)-9-nitro-7H-benzo[de]anthracen-7-one (4), Obtained as a red compound in a 59% yield with an m.p. of 271–273 °C. Rf = 0.02 (eluent C6H6-CH3CN, v/v 3:1). 1H NMR (500 MHz, CDCl3) δ 9.14 (d, J = 2.5 Hz, 1H, (8)), 8.72 (dd, J = 7.3, 1.4 Hz, 1H, (6)), 8.51 (dd, J = 8.3, 1.4 Hz, 1H, (4)), 8.34 (dd, J = 8.9, 2.6 Hz, 1H, (10)), 8.31 (d, J = 8.2 Hz, 1H, (1)), 8.23 (d, J = 9.0 Hz, 1H, (11)), 7.73 (dd, J = 8.3, 7.3 Hz, 1H, (5)), 7.18 (d, J = 8.2 Hz, 1H, (2)), 3.24 (t, J = 4.8 Hz, 4H, CH2), 2.71 (brs, 4H, CH2), 2.40 (s, 3H, CH3). 13C NMR (126 MHz, CDCl3) δ 182.26 (C=O), 154.66, 146.53, 141.43, 131.88 (CH, (4)), 130.81 (CH, (6)), 130.39, 129.75, 128.52, 127.93, 127.56 (CH, (1)), 126.80 (CH, (10)), 126.10 (CH, (5)), 123.89 (CH, (11)), 123.77 (CH, (8)), 119.33, 115.10 (CH, (2)), 77.28, 77.03, 76.77, 55.26, 53.35, 46.19. FTIR (neat): 402, 479, 505, 596, 654, 699, 749, 777, 828, 889, 925, 956, 1010, 1073, 1141, 1169, 1242, 1287, 1328, 1372, 1453, 1503, 1575, 1651, 2692, 2786, 2833, 2939, 3090. ESI-FTMS: calculated for [C22H19N3O3 + H+]: 374.1499, found: 374.1485.
- 9-Nitro-3-(piperidin-1-yl)-7H-benzo[de]anthracen-7-one (5), Obtained as a red compound in a 60% yield with an m.p. of 251–252 °C. Rf = 0.92 (eluent C6H6-CH3CN, v/v 3:1). 1H NMR (500 MHz, CDCl3) δ 9.13 (d, J = 2.5 Hz, 1H, (8)), 8.70 (d, J = 7.3 Hz, 1H, (6)), 8.48 (d, J = 8.3 Hz, 1H, (4)), 8.31 (dd, J = 8.9, 2.6 Hz, 1H, (10)), 8.27 (d, J = 8.2 Hz, 1H, (1)), 8.20 (d, J = 8.9 Hz, 1H, (11)), 7.71 (dd, J = 7.8 Hz, 1H), 7.11 (d, J = 8.2 Hz, 1H), 3.16 (s, 4H), 1.84 (p, J = 5.6 Hz, 4H), 1.67 (p, J = 5.8 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 182.30 (C=O), 156.09 (C), 146.32 (C), 141.57 (C), 132.21 (CH, (4)), 130.74 (CH, (6)), 130.23 (C), 129.76 (C), 128.44 (C), 128.05 (C), 127.70 (CH, (1)), 126.68 (CH, (10)), 125.85 (CH, (5)), 123.76 (CH, (11)), 123.76 (CH, (8)), 118.54 (C), 114.82 (CH, (2)), 54.93 (CH2, (2′, 6′)), 26.31 (CH2, (3′, 5′)), 24.39 (CH2, (4′)). FTIR (neat): 406, 452, 478, 530, 586, 619, 666, 697, 743, 774, 824, 888, 921, 952, 989, 1025, 1068, 1128, 1169, 1229, 1273, 1329, 1376, 1440, 1505, 1570, 1645, 2662, 2702, 2738, 2818, 2852, 2919, 3066, 3109, 3997. ESI-FTMS: calculated for [C22H18N2O3 + H+]: 359.1390, found: 359.1381.
- 3-(Piperidin-1-yl)-7H-benzo[de]anthracen-7-one (6), Obtained from 3-bromobenzanthrone at 120–130 °C for 6–7 h as an orange compound in a 48% yield with an m.p. of 165–166 °C. Rf = 0.94 (eluent C6H6-CH3CN, v/v 3:1). 1H NMR (500 MHz, CDCl3) δ 8.67 (d, J = 7.3 Hz, 1H, (8)), 8.46 (d, J = 8.3 Hz, 1H, (4)), 8.38 (d, J = 7.9 Hz, 1H, (6)), 8.21 (d, J = 8.1 Hz, 1H, (1)), 8.12 (d, J = 8.2 Hz, 1H, (11)), 7.65 (dd, J = 7.8 Hz, 1H, (5)), 7.58 (dd, J = 7.6 Hz, 1H, (10)), 7.38 (dd, J = 7.5 Hz, 1H, (9)), 7.06 (d, J = 8.0 Hz, 1H, (2)), 3.05 (brs, 4H, (2′, 6′)), 1.79 (p, J = 5.6 Hz, 4H, (3′, 5′)), 1.61 (brs, 2H, (4′)). 13C NMR (126 MHz, CDCl3) δ 184.15 (C=O), 153.93 (C), 136.60 (C), 133.16 (CH, (10)), 131.26 (CH, (4)), 130.24 (C), 129.76 (CH, (6)), 129.24 (C), 129.00 (C), 128.35 (C), 127.97 (CH, (8)), 127.16 (CH, (9)), 125.49 (CH, (5)), 125.14 (CH, (1)), 122.53 (CH, (11)), 120.97 (C), 114.88 (CH, (2)), 55.01 (CH2, (2′, 6′)), 26.46 (CH2, (3′, 5′)), 24.48 (CH2, (4′)). FTIR (neat): 410, 450, 473, 507, 581, 625, 653, 703, 772, 842, 878, 939, 961, 1027, 1060, 1101, 1168, 1206, 1277, 1375, 1463, 1511, 1573, 1643, 2668, 2704, 2737, 2808, 2847, 2930, 3064. ESI-FTMS: calculated for [C22H19NO + H+]: 314.1539, found: 314.1530.
3.3. Spectroscopic Measurements
3.4. Single Crystal X-ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zacharioudaki, D.-E.; Fitilis, I.; Kotti, M. Review of Fluorescence Spectroscopy in Environmental Quality Applications. Molecules 2022, 27, 4801. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, H.; Liang, Y.; Dai, H. Rational Design of High Brightness NIR-II Organic Dyes with S-D-A-D-S Structure. Acc. Mater. Res. 2021, 2, 170–183. [Google Scholar] [CrossRef]
- Mohd Yusof Chan, N.N.; Idris, A.; Zainal Abidin, Z.H.; Tajuddin, H.A.; Abdullah, Z. White Light Employing Luminescent Engineered Large (Mega) Stokes Shift Molecules: A Review. RSC Adv. 2021, 11, 13409–13445. [Google Scholar] [CrossRef]
- Wünsch, U.J.; Murphy, K.R.; Stedmon, C.A. Fluorescence Quantum Yields of Natural Organic Matter and Organic Compounds: Implications for the Fluorescence-Based Interpretation of Organic Matter Composition. Front. Mar. Sci. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Juette, M.F.; Jockusch, S.; Wasserman, M.R.; Zhou, Z.; Altman, R.B.; Blanchard, S.C. Ultra-Stable Organic Fluorophores for Single-Molecule Research. Chem. Soc. Rev. 2014, 43, 1044–1056. [Google Scholar] [CrossRef] [Green Version]
- Altaf, Y.; Ullah, S.; Khan, F.A.; Maalik, A.; Rubab, S.L.; Hashmi, M.A. Finding New Precursors for Light Harvesting Materials: A Computational Study of the Fluorescence Potential of Benzanthrone Dyes. ACS Omega 2021, 6, 32334–32341. [Google Scholar] [CrossRef] [PubMed]
- Grabchev, I.; Bojinov, V.; Moneva, I. The synthesis and application of fluorescent dyes based on 3-amino benzanthrone. Dyes Pigment. 2001, 48, 143–150. [Google Scholar] [CrossRef]
- Tsiko, U.; Sych, G.; Volyniuk, D.; Bezvikonnyi, O.; Keruckiene, R.; Lazauskas, A.; Grazulevicius, J.V. Self-recovering mechanochromic luminescence of the derivatives of benzanthrone and carbazole: Towards damage-resistive information recording and security probes. Dyes Pigment. 2022, 199, 110082. [Google Scholar] [CrossRef]
- Gavarane, I.; Kirilova, E.; Rubeniņa, I.; Mežaraupe, L.; Osipovs, S.; Deksne, G.; Pučkins, A.; Kokina, I.; Bulanovs, A.; Kirjušina, M. A Simple and Rapid Staining Technique for Sex Determination of Trichinella Larvae Parasites by Confocal Laser Scanning Microscopy. Microsc. Microanal. 2019, 25, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Rubenina, I.; Gavarane, I.; Kirilova, E.; Mezaraupe, L.; Kirjusina, M. Comparison of the Benzanthrone Luminophores: They Are Not Equal for Rapid Examination of Parafasciolopsis Fasciolaemorpha (Trematoda: Digenea). Biomolecules 2021, 11, 598. [Google Scholar] [CrossRef]
- Kirilova, E.; Mickevica, I.; Mezaraupe, L.; Puckins, A.; Rubenina, I.; Osipovs, S.; Kokina, I.; Bulanovs, A.; Kirjusina, M.; Gavarane, I. Novel Dye for Detection of Callus Embryo by Confocal Laser Scanning Fluorescence Microscopy. Luminescence 2019, 34, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Vus, K.; Trusova, V.; Gorbenko, G.; Sood, R.; Kirilova, E.; Kirilov, G.; Kalnina, I.; Kinnunen, P. Fluorescence Investigation of Interactions Between Novel Benzanthrone Dyes and Lysozyme Amyloid Fibrils. J. Fluoresc. 2014, 24, 493–504. [Google Scholar] [CrossRef]
- Tarabara, U.; Kirilova, E.; Kirilov, G.; Vus, K.; Zhytniakivska, O.; Trusova, V.; Gorbenko, G. Benzanthrone Dyes as Mediators of Cascade Energy Transfer in Insulin Amyloid Fibrils. J. Mol. Liq. 2021, 324, 115102. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I.; Wolarz, E.; Bauman, D. Fluorescent 3-Oxy Benzanthrone Dyes in Liquid Crystalline Media. Dyes Pigm 2003, 58, 1–6. [Google Scholar] [CrossRef]
- Grabtchev, I.K.; Bojinov, V.B.; Moneva, I.T. Functional Properties of Azomethine Substituted Benzanthrone Dyes for Use in Nematic Liquid Crystals. J. Mol. Struct. 1998, 471, 19–25. [Google Scholar] [CrossRef]
- Konstantinova, T.; Bojadgieva, J. On the Polymerization of Styrene in the Presence of Some Benzanthrone Dyes. Angew. Makromolek. Chem. 1993, 205, 91–95. [Google Scholar] [CrossRef]
- Konstantinova, T.N. The Synthesis of Some Benzanthrone Derivatives for Use as Dyes for Polymeric Materials. Dyes Pigment. 1989, 10, 63–67. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. PH Sensor Potential and Antimicrobial Activity of a New PPA Dendrimer Modified with Benzanthrone Fluorophores in Solution and on Viscose Fabric. J. Photochem. Photobiol. A Chem. 2019, 375, 24–29. [Google Scholar] [CrossRef]
- Maļeckis, A.; Avotiņa, L.; Ķizāne, G.; Pučkins, A.; Osipovs, S.; Kirilova, E. New Fluorescent Heterocyclic Compounds Derived From 3-Cyanobenzanthrone. Polycycl. Aromat. Compd. 2022, 42, 5508–5520. [Google Scholar] [CrossRef]
- Adam, A.M.A.; Altalhi, T.A.; El-Megharbel, S.M.; Saad, H.A.; Refat, M.S.; Grabchev, I.; Althobaiti, R.A. Capturing of Environment Polluting Metal Ions Co2+, Ni2+, Cu2+, and Zn2+ Using a 3-Azomethine Benzanthrone-Based Fluorescent Dye: Its Synthesis, Structural, and Spectroscopic Characterizations. Russ. J. Gen. Chem. 2020, 90, 2394–2399. [Google Scholar] [CrossRef]
- Bentley, P.; McKellar, J.F.; Phillips, G.O. The photochemistry of benz[de]anthracen-7-ones. Part I. Electronic absorption and emission spectroscopy. J. Chem. Soc. Perkin Trans. 2 1974, 5, 523–526. [Google Scholar] [CrossRef]
- Nepraš, M.; Machalický, O.; Šeps, M.; Hrdina, R.; Kapusta, P.; Fidler, V. Structure and properties of fluorescent reactive dyes: Electronic structure and spectra of some benzanthrone derivatives. Dyes Pigment. 1997, 35, 31–44. [Google Scholar] [CrossRef]
- Kapusta, P.; Machalicky, O.; Hrdina, R.; Nepras, M.; Zimmt, B.M.; Fidler, V. Photophysics of 3-substituted benzanthrones: Substituent and solvent control of intersystem crossing. J. Phys. Chem. 2003, 107, 9740–9746. [Google Scholar] [CrossRef]
- Orlova, N.; Nikolajeva, I.; Pučkins, A.; Belyakov, S.; Kirilova, E. Heterocyclic Schiff Bases of 3-Aminobenzanthrone and Their Reduced Analogues: Synthesis, Properties and Spectroscopy. Molecules 2021, 26, 2570. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. A New Bioactive Complex between Zn(II) and a Fluorescent Symmetrical Benzanthrone Tripod for an Antibacterial Textile. Materials 2019, 12, 3473. [Google Scholar] [CrossRef] [Green Version]
- Staneva, D.; Vasileva-Tonkova, E.; Makki, M.S.; Sobahi, T.R.; Abdel-Rahman, R.M.; Asiri, A.M.; Grabchev, I. Synthesis, photophysical and antimicrobial activity of new water soluble ammonium quaternary benzanthrone in solution and in polylactide film. J. Photochem. Photobiol. B 2015, 143, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kirilova, E.M.; Nikolaeva, I.D.; Romanovska, E.; Pučkins, A.I.; Belyakov, S.V. The Synthesis of Novel Heterocyclic 3-Acetamide Derivatives of Benzanthrone. Chem. Heterocycl. Compd. 2020, 56, 192–198. [Google Scholar] [CrossRef]
- Kirilova, E.M.; Puckins, A.I.; Romanovska, E.; Fleisher, M.; Belyakov, S.V. Novel Amidine Derivatives of Benzanthrone: Effect of Bromine Atom on the Spectral Parameters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 202, 41–49. [Google Scholar] [CrossRef]
- Maļeckis, A.; Griškjāns, E.; Cvetinska, M.; Savicka, M.; Belyakov, S.; Kirilova, E. Synthesis, Characterization, Spectroscopic Studies and Evaluation of Toxicological Effect on Growth of Wheat Sprouts (Triticum Aestivum) of New Benzanthrone α-Aryl-α-Aminophosphonates. J. Mol. Struct. 2023, 1277, 134838. [Google Scholar] [CrossRef]
- Thomas, A.; Patil, P.S.; Siddlingeshwar, B.; Manohara, S.R.; Gummagol, N.B.; Krishna Chaitanya, G.; M.Kirilova, E. Nonlinear Optical Properties of Benzanthrone Derivatives with N’-Methylpiperazin-1-Yl and N’-Phenylpiperazin-1-Yl Substituents: Experimental and Quantum Chemical Study. Opt. Laser Technol. 2022, 156, 108616. [Google Scholar] [CrossRef]
- Thomas, A.; Kirilova, E.M.; Nagesh, B.V.; Manohara, S.R.; Siddlingeshwar, B.; Belyakov, S.V. Synthesis, Solvatochromism and DFT Study of Pyridine Substituted Benzanthrone with ICT Characteristics. J. Mol. Struct. 2022, 1262, 132971. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, D.; Xu, L.; Fang, Z.; Jiang, X.-F.; Lu, F. Perylenediimide-Benzanthrone Dyad: Organic Chromophores with Enhanced Third-Order Nonlinear-Optical Activities. Eur. J. Org. Chem. 2017, 2017, 2495–2500. [Google Scholar] [CrossRef]
- Cao, L.; Xu, L.; Zhang, D.; Zhou, Y.; Zheng, Y.; Fu, Q.; Jiang, X.-F.; Lu, F. D-A Dyad and D-A-D Triad Incorporating Triphenylamine, Benzanthrone and Perylene Diimide: Synthesis, Electrochemical, Linear and Nonlinear Optical Properties. Chem. Phys. Lett. 2017, 682, 133–139. [Google Scholar] [CrossRef]
- Thomas, A.; Kirilova, E.M.; Nagesh, B.V.; Krishna Chaitanya, G.; Philip, R.; Manohara, S.R.; Sudeeksha, H.C.; Siddlingeshwar, B. Influence of Nitro Group on Solvatochromism, Nonlinear Optical Properties of 3-Morpholinobenzanthrone: Experimental and Theoretical Study. J. Photochem. Photobiol. A Chem. 2023, 437, 114434. [Google Scholar] [CrossRef]
- Day, F.H. Nitration of the 13-halogenobenzanthrones. J. Chem. Soc. 1940, 1474–1475. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Vaidyanathan, A. The 1H NMR Spectrum of Benzanthrone. Spectrochim. Acta A 1981, 37, 145–146. [Google Scholar] [CrossRef]
- Takekawa, M.; Aoki, J.; Iwashima, S.; Ueda, T. Complete Assignment Of1H And13C NMR Spectra of Chlorobenzanthrones. Magn. Reson. Chem. 1994, 32, 87–92. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ohshima, S.; Enya, T.; Suzuki, H.; Hisamatsu, Y. NMR Spectroscopy and Molecular Orbital Calculations to Interpret the Mutagenicity of Nitrobenzanthrones. Polycycl. Aromat. Compd. 2001, 19, 73–81. [Google Scholar] [CrossRef]
- Kirilova, E.M.; Belyakov, S.V.; Kirilov, G.K.; Kalnina, I.; Gerbreder, V. Luminescent properties and crystal structure of novel benzanthrone dyes. J. Luminesc. 2009, 129, 1827–1830. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Cryst. 2007, 40, 609–613. [Google Scholar] [CrossRef]





| δ, ppm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | ||||||
| 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | |
| C-8 | 9.19, d | 123.72 | 9.21, d | 124.07 | 9.14, d | 123.77 | 9.13, d | 123.76 | 8.67, d | 127.97 |
| C-4 | 8.55, d | 131.65 | 8.65, d | 133.24 | 8.51, dd | 131.88 | 8.48, d | 132.21 | 8.46, d | 131.26 |
| C-6 | 8.75, d | 130.86 | 8.78, d | 130.99 | 8.72, dd | 130.81 | 8.70, d | 130.74 | 8.38, d | 129.76 |
| C-10 | 8.39, d | 126.82 | 8.33, dd | 126.42 | 8.34, dd | 126.80 | 8.31, dd | 126.68 | 7.58, ddd | 133.16 |
| C-1 | 8.38, d | 127.46 | 8.26, d | 128.92 | 8.31, d | 127.56 | 8.27, d | 127.70 | 8.21, d | 125.14 |
| C-11 | 8.30, d | 123.94 | 8.20, d | 123.11 | 8.23, d | 123.89 | 8.20, d | 123.76 | 8.12, d | 122.53 |
| C-5 | 7.76, dd | 126.29 | 7.63, dd | 123.72 | 7.73, dd | 126.10 | 7.71, dd | 125.85 | 7.65, dd | 125.49 |
| C-2 | 7.21, d | 115.10 | 6.83, d | 108.86 | 7.18, d | 115.10 | 7.12, d | 114.82 | 7.06, d | 114.88 |
| C-7 | 182.17 | – | 182.26 | 182.30 | 184.15 | |||||
| C-9 | 7.38, ddd | 127.16 | ||||||||
| λabs max, nm (lgε) | ||||||
|---|---|---|---|---|---|---|
| Solvent | 3 | 7 | 2 | 4 | 5 | 6 |
| Benzene | 498 (3.94) | 525 (4.16) | 447 (4.47) | 453 (4.26) | 460 (4.55) | 447 (4.16) |
| Chloroform | 505 (4.01) | 541 (4.21) | 449 (4.56) | 462 (4.26) | 474 (4.62) | 454 (4.15) |
| EtOAc | 493 (3.92) | 527 (4.24) | 447 (4.49) | 454 (4.15) | 459 (4.53) | 445 (4.13) |
| Acetone | 512 (4.08) | 542 (4.31) | 448 (4.60) | 462 (4.11) | 467 (4.51) | 448 (4.12) |
| EtOH | 526 (3.97) | 542 (4.21) | 449 (4.49) | 455 (3.99) | 471 (4.50) | 457 (4.09) |
| DMF | 519 (4.18) | 548 (4.35) | 460 (4.54) | 467 (4.17) | 475 (4.50) | 448 (4.11) |
| DMSO | 531 (4.06) | 558 (4.31) | 466 (4.43) | 472 (4.11) | 478 (4.49) | 463 (4.11) |
| λem max, nm | ||||||
|---|---|---|---|---|---|---|
| Solvent | 3 | 7 | 2 | 4 | 6 | 5 |
| Benzene | 584 | 577 | 570 | 574 | 582 | 578 |
| Chloroform | 613 | 602 | 592 | 598 | 617 | 606 |
| EtOAc | 598 | 593 | 592 | 592 | 602 | 598 |
| Acetone | 621 | 632 | 612 | 612 | 628 | 620 |
| EtOH | 661 | 652 | 652 | 645 | 675 | 661 |
| DMF | 630 | 645 | 624 | 622 | 635 | 632 |
| DMSO | 641 | 645 | 650 | 634 | 665 | 643 |
| (νabs − νem) (cm−1) | ||||||
|---|---|---|---|---|---|---|
| Solvent | 3 | 7 | 2 | 4 | 6 | 5 |
| Benzene | 2957 | 1716 | 4564 | 4653 | 5189 | 4438 |
| Chloroform | 3489 | 1873 | 4827 | 4923 | 5819 | 4596 |
| EtOAc | 3562 | 2112 | 5380 | 5135 | 5861 | 5064 |
| Acetone | 3428 | 2627 | 5479 | 5305 | 6398 | 5284 |
| EtOH | 3882 | 3113 | 5981 | 6474 | 7067 | 6103 |
| DMF | 3395 | 2744 | 6935 | 5336 | 6573 | 5230 |
| DMSO | 3231 | 2417 | 5713 | 5413 | 6561 | 5368 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maļeckis, A.; Cvetinska, M.; Puckins, A.; Osipovs, S.; Sirokova, J.; Belyakov, S.; Kirilova, E. Synthesis and Properties of New 3-Heterylamino-Substituted 9-Nitrobenzanthrone Derivatives. Molecules 2023, 28, 5171. https://doi.org/10.3390/molecules28135171
Maļeckis A, Cvetinska M, Puckins A, Osipovs S, Sirokova J, Belyakov S, Kirilova E. Synthesis and Properties of New 3-Heterylamino-Substituted 9-Nitrobenzanthrone Derivatives. Molecules. 2023; 28(13):5171. https://doi.org/10.3390/molecules28135171
Chicago/Turabian StyleMaļeckis, Armands, Marija Cvetinska, Aleksandrs Puckins, Sergejs Osipovs, Jelizaveta Sirokova, Sergey Belyakov, and Elena Kirilova. 2023. "Synthesis and Properties of New 3-Heterylamino-Substituted 9-Nitrobenzanthrone Derivatives" Molecules 28, no. 13: 5171. https://doi.org/10.3390/molecules28135171
APA StyleMaļeckis, A., Cvetinska, M., Puckins, A., Osipovs, S., Sirokova, J., Belyakov, S., & Kirilova, E. (2023). Synthesis and Properties of New 3-Heterylamino-Substituted 9-Nitrobenzanthrone Derivatives. Molecules, 28(13), 5171. https://doi.org/10.3390/molecules28135171