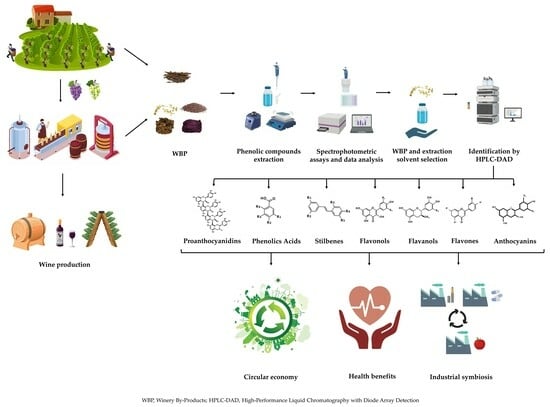
Exploring the Antioxidant Potential of Phenolic Compounds from Winery By-Products by Hydroethanolic Extraction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phenolic Content of Winery By-Products
2.2. Antioxidant Capacity of Winery By-Products
2.3. Principal Component Analysis
2.4. Phenolic Content and Antioxidant Capacity of Stem Varieties Hydroethanolic Extracts (50:50, v/v)
2.5. Phenolic Compound Identification in Hydroethanolic Stem Extracts (50:50, v/v) Using HPLC-DAD
2.5.1. Non-Flavonoids
Phenolic Acids
Stilbens
2.5.2. Flavonoids
Flavanols
Flavonols
Flavones
Anthocyanins
2.5.3. Proanthocyanidins
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sampling
3.3. Preparation of Winery By-Products Extracts
3.4. Determination of Phenolic Content
3.4.1. Total Phenols Content
3.4.2. Ortho-Diphenols Content
3.4.3. Flavonoids Content
3.5. Determination of Antioxidant Capacity
3.5.1. Antioxidant capacity by Ferric-Reducing Antioxidant Power (FRAP)
3.5.2. Antioxidant Capacity by DPPH
3.5.3. Antioxidant Capacity by ABTS
3.6. Identification of Phenolic Compounds by HPLC-DAD
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- OIV. Annual Assessment of the World Vine and Wine Sector in 2022; International Organization of Vine and Wine: Dijon, France, 2022; pp. 1–20. [Google Scholar]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; Dos Santos, L.F.; Franchin, M.; Pereira, G.E.; Vieira, T.M.F.D.S.; Rosalen, P.L.; De Alencar, S.M. Winery By-Products: Extraction Optimization, Phenolic Composition and Cytotoxic Evaluation to Act as a New Source of Scavenging of Reactive Oxygen Species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Domínguez-Perles, R.; Rodrigues, M.; Barros, A.I.R.N.A. Monitoring the Antioxidant and Antimicrobial Power of Grape (Vitis vinifera L.) Stems Phenolics over Long-Term Storage. Ind. Crops Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable Green Processing of Grape Pomace for the Production of Value-Added Products: An Overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive Compounds from Vine Shoots, Grape Stalks, and Wine Lees: Their Potential Use in Agro-Food Chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.I.; Moreno, L.; Espejo, A.; Machuca, S.; Almeida, T.; Santos, M.; Mondragão-Rodrigues, F.; Pacheco de Carvalho, G.; Paulo, M.; Sanchez, R. Valorização Dos Subprodutos Produzidos Nas Adegas Da Zona Euroace. 2020. Available online: https://www.rederural.gov.pt/centro-de-recursos/send/10-inovacao/1885-artigo-cientifico-valorizacao-dos-subprodutos-produzidos-nas-adegas-da-zona-euroace (accessed on 2 June 2022).
- Meloi, P.S.; Bergamaschi, K.B.; Tiveron, A.P.; Massarioli, A.P.; Oldoni, T.L.C.; Zanus, M.C.; Pereira, G.E.; de Alencar, S.M. Composição Fenólica e Atividade Antioxidante de Resíduos Agroindustriais. Cienc. Rural 2011, 41, 1088–1093. [Google Scholar] [CrossRef]
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and Chemical Variability of Grape Pomaces from French Vineyard. Ind. Crops Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Dias, C.; Domínguez-Perles, R.; Aires, A.; Teixeira, A.; Rosa, E.; Barros, A.; Saavedra, M.J. Phytochemistry and Activity against Digestive Pathogens of Grape (Vitis vinifera L.) Stem’s (Poly)Phenolic Extracts. LWT-Food Sci. Technol. 2015, 61, 25–32. [Google Scholar] [CrossRef]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential Application of Grape (Vitis vinifera L.) Stem Extracts in the Cosmetic and Pharmaceutical Industries: Valorization of a by-Product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and Future Strategies for Wine Yeast Lees Valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.M.; Faccin, H.; Viana, C.; da Rosa, M.B.; de Carvalho, L.M. Vitis Vinifera L. Cv Pinot Noir Pomace and Lees as Potential Sources of Bioactive Compounds. Int. J. Food Sci. Nutr. 2016, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Gouvinhas, I.; Pinto, R.; Santos, R.; Saavedra, M.J.; Barros, A.I. Enhanced Phytochemical Composition and Biological Activities of Grape (Vitis vinifera L.) Stems Growing in Low Altitude Regions. Sci. Hortic. 2020, 265, 109248. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery By-Products: Extraction Techniques and Their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Medina, S.; Sánchez-Bravo, P.; Domínguez-Perles, R. The (Poly) Phenolic Profile of Separate Winery By-Products Reveals Potential Antioxidant Synergies. Molecules 2023, 28, 2081. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic Content and in Vitro Antioxidant Characteristics of Wine Industry and Other Agri-Food Solid Waste Extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of Grape Stems as a Source of Phenolic Antioxidants by Using a Sustainable Extraction Methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese Vine Shoot Wastes as Natural Resources of Bioactive Compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J.L. Valorisation Potential of Cabernet Grape Pomace for the Recovery of Polyphenols: Process Intensification, Optimisation and Study of Kinetics. Food Bioprod. Process. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.D.M. Extraction Systems and Analytical Techniques for Food Phenolic Compounds: A Review. Foods 2022, 11, 3671. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Teixeira, A.I.; Rosa, E.; Barros, A.I. Assessment of (Poly)Phenols in Grape (Vitis vinifera L.) Stems by Using Food/Pharma Industry Compatible Solvents and Response Surface Methodology. Food Chem. 2014, 164, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Spigno, G.; De Faveri, D.M. Antioxidants from Grape Stalks and Marc: Influence of Extraction Procedure on Yield, Purity and Antioxidant Power of the Extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancín-Azpilicueta, C. Impact of Extraction Conditions on the Phenolic Composition and Antioxidant Capacity of Grape Stem Extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef]
- Pinton, S.; Furlan Goncalves Dias, F.; Lerno, L.A.; Barile, D.; Leite Nobrega de Moura Bell, J.M. Revitalizing Unfermented Cabernet Sauvignon Pomace Using an Eco-Friendly, Two-Stage Countercurrent Process: Role of PH on the Extractability of Bioactive Phenolics. Processes 2022, 10, 2093. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Chiou, A.; Andrikopoulos, N.K. An Investigation on Factors Affecting Recovery of Antioxidant Phenolics and Anthocyanins from Red Grape (Vitis vinifera L.) Pomace Employing Water/Ethanol-Based Solutions. Am. J. Food Technol. 2008, 3, 164–173. [Google Scholar] [CrossRef]
- Garrido, T.; Gizdavic-Nikolaidis, M.; Leceta, I.; Urdanpilleta, M.; Guerrero, P.; de la Caba, K.; Kilmartin, P.A. Optimizing the Extraction Process of Natural Antioxidants from Chardonnay Grape Marc Using Microwave-Assisted Extraction. Waste Manag. 2019, 88, 110–117. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of Grape Pomace: Extraction of Bioactive Phenolics with Antioxidant Properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Medouni-Adrar, S.; Boulekbache-Makhlouf, L.; Cadot, Y.; Medouni-Haroune, L.; Dahmoune, F.; Makhoukhe, A.; Madani, K. Optimization of the Recovery of Phenolic Compounds from Algerian Grape By-Products. Ind. Crops Prod. 2015, 77, 123–132. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen Radical Absorbance Capacities of Grape/Wine Industry Byproducts and Effect of Solvent Type on Extraction of Grape Seed Polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Göktürk Baydar, N. Chemical Composition of Grape Canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A Comparative Study of Physical Pretreatments for the Extraction of Polyphenols and Proteins from Vine Shoots. Food Res int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; García-Moreno, M.V.; Igartuburu, J.M.; Garcia Barroso, C. Simplification of the DPPH Assay for Estimating the Antioxidant Activity of Wine and Wine By-Products. Food Chem. 2014, 165, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Brazinha, C. Fractionation of Hydro-Ethanolic Extracts from Grape Pomace through Membrane Processing: The Effect of Membrane and Extracting Media on Process Performance. 2014. Available online: https://run.unl.pt/handle/10362/13289 (accessed on 14 May 2023).
- Spatafora, C.; Barbagallo, E.; Amico, V.; Tringali, C. Grape Stems from Sicilian Vitis Vinifera Cultivars as a Source of Polyphenol-Enriched Fractions with Enhanced Antioxidant Activity. LWT-Food Sci. Technol. 2013, 54, 542–548. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. A Potential Valorization Strategy of Wine Industry By-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef]
- Sette, P.; Rodriguez, R.; Salvatori, D.; Fernandez, A. Integral Valorization of Fruit Waste from Wine and Cider Industries n Mazza. J. Clean. Prod. 2020, 242, 118486. [Google Scholar] [CrossRef]
- Pedras, B.; Salema-Oom, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Valorization of White Wine Grape Pomace through Application of Subcritical Water: Analysis of Extraction, Hydrolysis, and Biological Activity of the Extracts Obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the Extraction of Polyphenols from Grape Seed Meal by Aqueous Ethanol Solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of Phenolics from Vitis Vinifera Wastes Using Non-Conventional Techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Bilić, M.; Velić, D. Study of Solid–Liquid Extraction Kinetics of Total Polyphenols from Grape Seeds. J. Food Eng. 2007, 81, 236–242. [Google Scholar] [CrossRef]
- Dent, M.; Dragovi, V.; Peni, M.; Brn, M. The Effect of Extraction Solvents, Temperature and Time on the Composition and Mass Fraction of Polyphenols in Dalmatian Wild Sage (Salvia officinalis L.) Extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and Ultrasound Pre-Treatments to Enhance Anthocyanins Extraction from Different Wine Lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic Characterization of Aging Wine Lees: Correlation with Antioxidant Activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, M.G.; Francavilla, M.; Albenzio, M.; Inghese, C.; Santillo, A.; Sevi, A.; Caroprese, M. Green Extraction of Bioactive Compounds from Wine Lees and Their Bio-Responses on Immune Modulation Using in Vitro Sheep Model. J. Dairy Sci. 2022, 105, 4335–4353. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in Grape Seeds-Biochemistry and Functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Goldstein, D.B.; Chin, J.H. Interaction of Ethanol with Biological Membranes. Fed. Proc. 1981, 40, 2073–2076. [Google Scholar] [PubMed]
- Alexandru, L.; Binello, A.; Mantegna, S.; Boffa, L.; Chemat, F.; Cravotto, G. Comptes Rendus Chimie Efficient Green Extraction of Polyphenols from Post-Harvested Agro-Industry Vegetal Sources in Piedmont. Comptes Rendus-Chim. 2014, 17, 212–217. [Google Scholar] [CrossRef]
- Schechtel, S.L.; de Matos, V.C.R.; Santos, J.S.; Cruz, T.M.; Marques, M.B.; Wen, M.; Zhang, L.; Furtado, M.M.; Sant’Ana, A.S.; Vieira do Carmo, M.A.; et al. Flaxleaf Fleabane Leaves (Conyza Bonariensis), A New Functional Nonconventional Edible Plant? J. Food Sci. 2019, 84, 3473–3482. [Google Scholar] [CrossRef]
- Escher, G.B.; Borges, L.D.C.C.; Santos, J.S.; Cruz, T.M.; Marques, M.B.; Do Carmo, M.A.V.; Azevedo, L.; Furtado, M.M.; Sant’ana, A.S.; Wen, M.; et al. From the Field to the Pot: Phytochemical and Functional Analyses of Calendula officinalis L. Flower for Incorporation in an Organic Yogurt. Antioxidants 2019, 8, 559. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine By-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.; Haroutounian, S.A. Grape Stem Extracts: Polyphenolic Content and Assessment of Their in Vitro Antioxidant Properties. LWT-Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Sun, B.; Ribes, A.M.; Conceic, M.; Belchior, A.P.; Spranger, M.I. Stilbenes: Quantitative Extraction from Grape Skins, Contribution of Grape Solids to Wine and Variation during Wine Maturation. Anal. Chim. Acta 2006, 563, 382–390. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-vilaplana, A.; Teixeira, A.; Collado-gonzález, J.; Moreno, D.A.; Gil-izquierdo, A.; Rosa, E.; Domínguez-perles, R. Evaluation of Grape (Vitis vinifera L.) Stems from Portuguese Varieties as a Resource of (Poly) Phenolic Compounds: A Comparative Study. Food Res. Int. 2014, 65, 375–384. [Google Scholar] [CrossRef]
- Esparza, I.; Moler, J.A.; Arteta, M.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages. Biomolecules 2021, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Prusova, B.; Licek, J.; Kumsta, M.; Baron, M.; Sochor, J. Polyphenolic Composition of Grape Stems. Not. Bot. Horti Agrobot. 2020, 48, 1543–1560. [Google Scholar] [CrossRef]
- Souquet, J.-M.; Labarbe, B.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. Phenolic Composition of Grape Stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef]
- Abraão, A.S.; Fernandes, N.; Silva, A.M.; Domínguez-Perles, R.; Barros, A. Prunus Lusitanica L. Fruits as a Novel Source of Bioactive Compounds with Antioxidant Potential: Exploring the Unknown. Antioxidants 2022, 11, 1738. [Google Scholar] [CrossRef]
- Santos, S.; Maia, M.; Barros, A.; Gouvinhas, I. Assessment of Phenolic Content, Antioxidant and Anti-Aging Activities of Honey from Pittosporum Undulatum Vent. Naturalized in the Azores Archipelago (Portugal). Appl. Sci. 2023, 13, 1788. [Google Scholar] [CrossRef]









| Spectrophotometric Assays | White-Stem Varieties | Red-Stem Varieties | ||||
|---|---|---|---|---|---|---|
| F | CL | V | MF | TF | TR | |
| TPC (mg GA/g DW) | 59.51 ± 3.88 ab | 60.35 ± 1.63 ab | 64.43 ± 1.34 a | 61.54 ± 1.46 ab | 56.17 ± 1.16 b | 64.73 ± 1.40 a |
| ODC (mg GA/g DW) | 50.65 ± 0.93 b | 53.30 ± 2.29 ab | 55.73 ± 0.68 ab | 58.93 ± 4.17 a | 54.08 ± 0.81 ab | 51.69 ± 0.80 b |
| FC (mg CAT/g DW) | 35.62 ± 1.15 b | 36.89 ± 1.93 b | 45.09 ± 1.09 a | 40.66 ± 3.01 ab | 40.52 ± 0.05 ab | 43.76 ± 3.92 a |
| FRAP (mmol T/g DW) | 0.55 ± 0.01 c | 0.56 ± 0.02 bc | 0.62 ± 0.01 a | 0.59 ± 0.02 abc | 0.55 ± 0.01 c | 0.61 ± 0.01 ab |
| DPPH (mmol T/g DW) | 0.46 ± 0.01 c | 0.51 ± 0.01 bc | 0.58 ± 0.04 ab | 0.60 ± 0.03 a | 0.55 ± 0.04 ab | 0.56 ± 0.03 ab |
| ABTS (mmol T/g DW) | 0.51 ± 0.01 b | 0.53 ± 0.01 b | 0.58 ± 0.00 a | 0.57 ± 0.00 a | 0.57 ± 0.01 a | 0.58 ± 0.01 a |
| Class of Compounds | Peak Number * | Rt | Identified Compounds | Vitis vinifera L. Stems Varieties | |||||
|---|---|---|---|---|---|---|---|---|---|
| F | CL | V | MF | TF | TR | ||||
| Phenolic Acids | 1 | 6.9 | Protocatechuic acid hexoside | X | X | <LOD Y | X | X | X |
| 3 | 8.9 | trans-caftaric acid | X | X | X | X | X | X | |
| Stilbenes | 16 | 17.1 | Oxyresveratrol | X | X | X | X | <LOD | X |
| 19 | 17.8 | trans-piceid | <LOD | <LOD | <LOD | X | X | X | |
| 26 | 22.8 | ԑ-viniferin | X | X | X | X | <LOD | X | |
| Flavanols | 6 | 10.2 | Catechin | X | X | X | X | X | X |
| 12 | 15.4 | Epicatechin gallate | X | X | X | X | <LOD | X | |
| Flavonols | 9 | 11.8 | Quercetin-glucoside | <LOD | <LOD | <LOD | X | <LOD | X |
| 11 | 15.3 | Quercetin-3-rutinoside | X | X | X | X | X | X | |
| 13 | 16.0 | Quercetin-3-O-glucuronide | X | X | X | X | X | X | |
| 14 | 16.4 | Kaempferol-3-O-glucoside | X | X | X | X | <LOD | <LOD | |
| 18 | 17.6 | Kaempferol-7-O-β-d-glucopyranoside | <LOD | <LOD | <LOD | X | X | X | |
| Flavones | 15 | 16.6 | Luteolin-rutinoside | X | <LOD | X | X | <LOD | <LOD |
| Anthocyanins | 17 | 17.4 | Delphinidin-3-O-glucoside | <LOD | <LOD | <LOD | X | ||
| 20 | 19.0 | Cyanidin-3-O-glucoside | <LOD | <LOD | <LOD | <LOD | <LOD | X | |
| 22 | 20.0 | Petunidin-3-O-glucoside | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| 23 | 21.7 | Peonidin-3-O-glucoside W | <LOD | <LOD | <LOD | <LOD | X | X | |
| 24 | 21.7 | Malvidin-3-O-glucoside W | <LOD | <LOD | <LOD | <LOD | X | X | |
| 25 | 22.1 | Delphinidin-3-O-acetylglucoside | <LOD | <LOD | <LOD | <LOD | X | X | |
| 27 | 27.8 | Peonidin-3-O-acetylglucoside | <LOD | <LOD | <LOD | <LOD | X | X | |
| 28 | 27.8 | Malvidin-3-O-acetylglucoside | <LOD | <LOD | <LOD | <LOD | X | X | |
| 29 | 29.4 | Delphinidin-3-O-p-coumaroylglucoside | <LOD | <LOD | <LOD | <LOD | X | X | |
| 30 | 29.9 | Cyanidin-3-O-p-coumaroylglucoside | <LOD | <LOD | <LOD | <LOD | X | X | |
| 31 | 31.0 | Petunidin-3-O-p-coumaroylglucoside Z | <LOD | <LOD | <LOD | <LOD | X | X | |
| 32 | 31.0 | Malvidin-3-O-p-coumaroylglucoside Z | <LOD | <LOD | <LOD | <LOD | X | X | |
| Proanthocyanidins | 2 | 8.2 | Proanthocyanidin dimer (B-type) Isomer 1 | X | X | X | X | X | X |
| 4 | 9.5 | Proanthocyanidin dimer (B-type) Isomer 2 | <LOD | <LOD | X | X | <LOD | X | |
| 5 | 9.8 | Proanthocyanidin trimer (B-type) Isomer 1 | X | X | X | X | <LOD | <LOD | |
| 7 | 10.6 | Proanthocyanidin dimer-gallate Isomer 1 | X | X | X | X | X | X | |
| 8 | 11.2 | Proanthocyanidin dimer-gallate Isomer 2 | X | X | X | X | X | X | |
| 10 | 12.1 | Proanthocyanidin trimer (B-type) Isomer 2 | X | <LOD | X | X | X | <LOD | |
| 21 | 19.5 | Proanthocyanidin trimmer (B-type) monogallate | X | X | X | X | X | X | |
| WBP | Samples Number | Type of Variety | Varieties | Harvest | Sampling Step | Sub-Regions |
|---|---|---|---|---|---|---|
| Stems | 3 | Single white variety | F, V, MF | 2021 | After destemming | CC |
| 1 | Single white variety | CL | 2021 | BC | ||
| 2 | Single red varieties | TF, TR | 2021 | CC | ||
| Pomace (Seeds, pulp, skins) | 2 | Single white variety | M | 2021 | Before fermentation | BC |
| Single white variety | F | 2021 | CC | |||
| 1 | Mixture of several red varieties | TN, TF, So | 2021 | After fermentation | DS | |
| Seeds | 2 | Single white variety | M | 2022 | Before fermentation | BC |
| Mixture of several white varieties | Vio, MF, FP | 2022 | BC, CC, DS | |||
| 2 | Mixture of several red varieties | TN, TR, TB | 2022 | After fermentation | BC, CC, DS | |
| Mixture of several red varieties | TN, TB, TA, TR | 2022 | CC | |||
| Wine lees | 1 | Mixture of several white varieties | R, MF, Vio | 2022 | Post alcoholic fermentation | BC, CC, DS |
| 1 | Mixture of several red varieties | TN, TF, TR | 2022 | BC, CC, DS | ||
| Grapevine shoots | 10 | Single white varieties | S, F, R, EC, A, Vio, CL, MR, MF, FP | 2021 | After pruning (lignified) | BC |
| 3 | Single red varieties | TA, TR, TB | 2021 | BC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, R.D.; Domínguez-Perles, R.; Abraão, A.; Gomes, V.; Gouvinhas, I.; Barros, A.N. Exploring the Antioxidant Potential of Phenolic Compounds from Winery By-Products by Hydroethanolic Extraction. Molecules 2023, 28, 6660. https://doi.org/10.3390/molecules28186660
Costa RD, Domínguez-Perles R, Abraão A, Gomes V, Gouvinhas I, Barros AN. Exploring the Antioxidant Potential of Phenolic Compounds from Winery By-Products by Hydroethanolic Extraction. Molecules. 2023; 28(18):6660. https://doi.org/10.3390/molecules28186660
Chicago/Turabian StyleCosta, Rui Dias, Raúl Domínguez-Perles, Ana Abraão, Véronique Gomes, Irene Gouvinhas, and Ana Novo Barros. 2023. "Exploring the Antioxidant Potential of Phenolic Compounds from Winery By-Products by Hydroethanolic Extraction" Molecules 28, no. 18: 6660. https://doi.org/10.3390/molecules28186660
APA StyleCosta, R. D., Domínguez-Perles, R., Abraão, A., Gomes, V., Gouvinhas, I., & Barros, A. N. (2023). Exploring the Antioxidant Potential of Phenolic Compounds from Winery By-Products by Hydroethanolic Extraction. Molecules, 28(18), 6660. https://doi.org/10.3390/molecules28186660