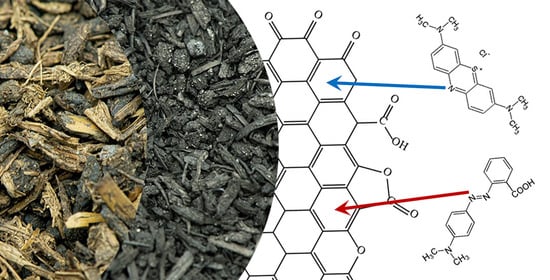
Adsorption of Methyl Red and Methylene Blue on Carbon Bioadsorbents Obtained from Biogas Plant Waste Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical, Acid-Base Properties of the Precursor, Pyrolysis Product and Biocarbons Obtained
2.2. Adsorption Study
3. Materials and Methods
3.1. Precursor and Biocarbons
3.2. Precursor/Biocarbons Characteristics
3.3. Adsorption of Methylene Blue/Methyl Red
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Desore, A.; Narula, S.A. An overview on corporate response towards sustainability issues in textile industry. Environ. Dev. Sustain. 2018, 20, 1439–1459. [Google Scholar] [CrossRef]
- Amarzadeh, M.; Azqandi, M.; Nateq, K.; Ramavandi, B.; Khan, N.A.; Nasseh, N. Heterogeneous Fenton-like Photocatalytic Process towards the Eradication of Tetracycline under UV Irradiation: Mechanism Elucidation and Environmental Risk Analysis. Water 2023, 15, 2336. [Google Scholar] [CrossRef]
- Hossain, M.S.; Das, S.C.; Islam, J.M.; Al Mamun, M.A.; Khan, M.A. Reuse of textile mill ETP sludge in environmental friendly bricks-effect of gamma radiation. Radiat. Phys. Chem. 2018, 151, 77–83. [Google Scholar] [CrossRef]
- Wang, D.M. Environmental protection in clothing industry. In Sustainable Development: Proceedings of the 2015 International Conference on Sustainable Development, New York City, NY, USA, 23–24 September 2015; Zhu, L., Ed.; World Scientific Publishing Co. Pte Ltd.: Singapore, 2016; pp. 729–735. [Google Scholar]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Crowley, D.E.; Khalid, A.; Hussain, S.; Mumtaz, M.W.; Arshad, M. Microbial biotechnology for decolorization of textile wastewaters. Rev. Environ. Sci. Biotechnol. 2015, 14, 73–92. [Google Scholar] [CrossRef]
- Moghaddama, N.S.M.; Barikbin, B.; Al-Essac, E.M.; Khosravid, R.; Al-Musawie, T.; Nassehf, N. Application of magnetic activated carbon coated with CuS nanoparticles as a new adsorbent for the removal of tetracycline antibiotic from aqueous solutions (isotherm, kinetic and thermodynamic study). Desalin. Water Treat. 2022, 280, 297–311. [Google Scholar] [CrossRef]
- Hossein Panahi, A.; Al-Musawi, T.J.; Masihpour, M.; Fard, S.F.T.; Nasseh, N. Photocatalytic Degradation of Humic Acid Using Bentonite@Fe3O4@ZnO Magnetic Nanocomposite: An Investigation of the Characterization of the Photocatalyst, Degradation Pathway, and Modeling by Solver Plugin. Water 2023, 15, 2931. [Google Scholar] [CrossRef]
- Nasseha, N.; Al-Musawi, T.J.; Khosravi, R.; Panahi, A.H.; Arghavan, F.S.; Barikbin, B. FeNi3@SiO2@CuS magnetic nanocomposite: Synthesizing, characterization, and application for methylene blue adsorption. Desalin. Water Treat. 2021, 210, 402–414. [Google Scholar] [CrossRef]
- Khatri, J.; Nidheesh, P.V.; Singh, T.A.; Kumar, M.S. Advanced oxidation processes based on zero-valent aluminium for treating textile wastewater. Chem. Eng. J. 2018, 348, 67–73. [Google Scholar] [CrossRef]
- Sandhya, S. Biodegradation of azo dyes under anaerobic condition: Role of azoreductase. In Biodegra-Dation of Azo Dyes; The Handbook of Environmental Chemistry; Erkurt, H.A., Ed.; Springer: Berlin, Germany, 2010; Volume 9, pp. 39–57. [Google Scholar]
- Rehman, K.; Shahzad, T.; Sahar, A.; Hussain, S.; Mahmood, F.; Siddique, M.H.; Siddique, M.A.; Rashid, M.I. Effect of Reactive Black 5 azodye on soil processes related to C and N cycling. PeerJ 2018, 6, e4802. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Saha, B.; Mazloum, S.; Taylor, K.E.; Bewtra, J.K.; Biswas, N. Soybean Peroxidase. In Applications in Wastewater Treatment, 6th ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011. [Google Scholar]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ludin Norasikin, A.A.; Mohamad, A.; Kadhum, A.A.H.; Mukhlus, A. Application of dyes extracted from Alternanthera Dentata leaves and Musa Acuminata Bracts as natural sensitizers for dye-sensitized solar cells. Spectrochim. Acta Part A Mol. Biomol. 2018, 192, 487–498. [Google Scholar] [CrossRef]
- Solís, M.; Solís, A.; Pérez, H.I.; Manjarrez, N.; Flores, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar] [CrossRef]
- Santos, A.B.; Cervantes, F.J.; van Lier, J.B. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresour. Technol. 2007, 98, 2369–2385. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Zhao, G.; Song, G.; Chen, D.; Chen, H.; Xie, J.; Hayat, T.; Alsaedi, A.; Wang, X. Polyvinylpyrrolidone and polyacrylamide intercalated molybdenum disulfide as adsorbents for enhanced removal of chromium(VI) from aqueous solutions. Chem. Eng. J. 2018, 334, 569–578. [Google Scholar] [CrossRef]
- Khan, N.A.; Bhadra, B.N.; Jhung, S.H. Heteropoly acid loaded ionic liquid@metal-organic frameworks: Effective and reusable adsorbents for the desulfurization of a liquid model fuel. Chem. Eng. J. 2018, 334, 2215–2221. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Wan, A.K.; Wan, A.A.; Azni Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Angela Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M. Anion exchange resins as effective sorbents for acidic dye removal from aqueous solutions and wastewaters. Solvent Extr. Ion Exch. 2012, 30, 507–523. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Hosseini, S.S.; Akbari, A.; Ramavandi, B. Hydroxyapatite biomaterial production from chicken (femur and beak) and fishbone waste through a chemical less method for Cd2+ removal from shipbuilding wastewater. J. Hazard. Mater. 2021, 413, 125428. [Google Scholar] [CrossRef] [PubMed]
- Ambika, S.; Srilekha, V. Eco-safe chemicothermal conversion of industrial graphite waste to exfoliated graphene and evaluation as engineered adsorbent to remove toxic textile dyes. Environ. Adv. 2021, 4, 1000072. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hamacha, R.; Morsli, A.; Bengueddach, A. Adsorption of yellow dye on calcined or uncalcined Al-MCM-41 mesoporous materials. Arab. J. Chem. 2017, 10, S2160–S2169. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Pietrzak, R. Adsorption of organic and inorganic pollutants on activated bio-carbons prepared by chemical activation of residues of supercritical extraction of raw plants. Chem. Eng. J. 2020, 393, 124785. [Google Scholar] [CrossRef]
- Farahani, B.V.; BehbahanI, G.R.; Javad, N. Functionalized multi walled carbon nanotubes as a carrier for doxorubicin: Drug adsorption study and statistical optimization of drug loading by factorial design methodology. J. Braz. Chem. Soc. 2016, 27, 694–705. [Google Scholar] [CrossRef]
- Ismail, M.S.; Yahya, M.D.; Auta, M.; Obayomi, K.S. Facile preparation of amine-functionalized corn husk derived activated carbon for effective removal of selected heavy metals from battery recycling wastewater. Heliyon 2022, 8, e09516. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Pietrzak, R. Adsorption of cationic dye on nanostructured biocarbons: Kinetic and thermodynamic study. Appl. Nanosci. 2023, 1–15. [Google Scholar] [CrossRef]
- Güzel, F.; Sayğılı, H.; Sayğılı, G.A.; Koyuncu, F.; Yılmaz, C. Optimal oxidation with nitric acid of biochar derived from pyrolysis of weeds and its application in removal of hazardous dye methylene blue from aqueous solution. J. Clean. Prod. 2017, 144, 260–265. [Google Scholar] [CrossRef]
- Tan, C.H.C.; Sabar, S.; Hussin, M.H. Development of immobilized microcrystalline cellulose as an effective adsorbent for methylene blue dye removal. S. Afr. J. Chem. Eng. 2018, 26, 11–24. [Google Scholar] [CrossRef]
- da Silva Andrade, J.G.; Porto, C.E.; Moreira, W.M.; Batistela, V.R.; Olsen Scaliante, M.H.N. Production of hydrochars from Pinus caribaea for biosorption of methylene blue and tartrazine yellow dyes. CLCE 2023, 5, 100092. [Google Scholar] [CrossRef]
- Gul, S.; Kanwal, M.; Qazi, R.A.; Gul, H.; Khattak, R.; Khan, M.S.; Khitab, F.; Krauklis, A.E. Efficient Removal of Methyl Red Dye by Using Bark of Hopbush. Water 2022, 14, 2831. [Google Scholar] [CrossRef]
- Enenebeaku, C.K.; Okorocha, N.J.; Uchechi, E.E.; Ukaga, I.C. Adsorption and equilibrium studies on the removal of methyl red from aqueous solution using white potato peel powder. Int. Lett. Chem. Phys. Astron. 2017, 72, 52. [Google Scholar] [CrossRef]
- Lafi, R.; Abdellaoui, L.; Montasser, I.; Mabrouk, W.; Hafiane, A. The effect of head group of surfactant on the adsorption of methyl red onto modified coffee residues. J. Mol. Struct. 2022, 1249, 131527. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Nowicki, P.; Pietrzak, R. The effect of demineralization on the physicochemical and sorption properties of activated bio-carbons. Adsorption 2019, 25, 337–343. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of Point of Zero Charge of Natural Organic Materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef]
- Du, Z.D.; Cui, Y.Y.; Yang, C.X. Core-shell magnetic amino-functionalized microporous organic network nanospheres for the removal of tetrabromobisphenol A from aqueous solution. ACS Appl. Nano Mater. 2019, 2, 1232–1241. [Google Scholar] [CrossRef]
- Zhang, M.M.; Yu, H.C.; Chen, B.B. Facile synthesis of EDTA-functionalized halloysite nanotubes for the removal of methylene blue from aqueous phase. Can. J. Chem. 2019, 97, 259–266. [Google Scholar] [CrossRef]
- Reddy, Y.S.; Rotte, N.K.; Hussain, S.; Srikanth, V.V.S.S.; Chandra, M.R. Sustainable mesoporous graphitic activated carbon as biosorbent for efficient adsorption of acidic and basic dyes from wastewater: Equilibrium, kinetics and thermodynamic studies. J. Hazard. Mater. Adv. 2023, 9, 100214. [Google Scholar] [CrossRef]










| Sample | Ash | Cdaf 1 | Hdaf | Ndaf | Sdaf | Odiff 2 | Yield 3 |
|---|---|---|---|---|---|---|---|
| Precursor | 27.4 | 43.2 | 6.5 | 3.5 | 1.6 | 45.2 | - |
| P | 55.8 | 81.3 | 3.2 | 4.5 | 2.3 | 8.7 | 59.8 |
| PA6 | 49.6 | 74.6 | 1.8 | 3.6 | 1.3 | 18.7 | 54.4 |
| PA7 | 52.7 | 59.4 | 1.6 | 3.2 | 1.6 | 34.2 | 50.8 |
| Sample | Acidic Group Content [mmol/g] | Basic Group Content [mmol/g] | Total Content of Surface Groups [mmol/g] | pH of Aqueous Extracts |
|---|---|---|---|---|
| Precursor | 1.35 | 0.44 | 1.79 | 4.29 |
| PA6 | 0.92 | 0.31 | 1.23 | 5.64 |
| PA7 | 0.69 | 0.42 | 1.11 | 5.96 |
| Sample | Total 1 | Micropore | Pore Size [nm] | ||
|---|---|---|---|---|---|
| Surface Area [m2/g] | Pore Volume [cm3/g] | Area [m2/g] | Volume [cm3/g] | ||
| PA6 | 320 | 0.23 | 276 | 0.18 | 3.96 |
| PA7 | 616 | 0.48 | 490 | 0.40 | 3.20 |
| Model | Parameters | Methylene Blue | Methyl Red | ||
|---|---|---|---|---|---|
| PA6 | PA7 | PA6 | PA7 | ||
| qe [mg/g] | 40 | 146 | 31 | 113 | |
| Langmuir | qm [mg/g] | 41 | 147 | 33 | 115 |
| R2 | 0.999 | 0.993 | 0.990 | 0.991 | |
| RL | 0.116–0.316 | 0.502–0.729 | 0.512–0.759 | 0.493–0.722 | |
| KL [L/mg] | 0.216 | 0.016 | 0.032 | 0.013 | |
| Freundlich | R2 | 0.940 | 0.990 | 0.959 | 0.981 |
| KF [mg/g(L/mg)1/n] | 31.20 | 94.17 | 13.78 | 52.88 | |
| 1/n | 0.132 | 0.179 | 0.330 | 0.293 | |
| Model | Parameters | Methylene Blue | Methyl Red | ||
|---|---|---|---|---|---|
| PA6 | PA7 | PA6 | PA7 | ||
| qt [mg/g] | 22 | 83 | 31 | 50 | |
| Pseudo-first order | qe,cal [mg/g] | 5 | 14 | 34 | 13 |
| R2 | 0.961 | 0.949 | 0.9730 | 0.885 | |
| k1 [1/min] | 3.77 × 10−2 | 2.40 × 10−2 | 1.17 × 10−2 | 9.90 × 10−3 | |
| Pseudo-second order | qe,cal [mg/g] | 22 | 84 | 33 | 51 |
| R2 | 1 | 0.999 | 0.994 | 0.999 | |
| k2 [g/mg × min] | 2.25 × 10−2 | 2.19 × 10−3 | 9.96 × 10−4 | 2.70 × 10−3 | |
| Biocarbon | qe [mg/g] | Temperature [K] | ∆G0 [kJ/mol] | ∆H0 [kJ/mol] | ∆S0 [J/mol K] |
|---|---|---|---|---|---|
| PA6 (methylene blue) | 37 | 298 | −4.59 | 9.53 | 40.11 |
| 40 | 318 | −5.50 | |||
| 47 | 338 | −6.49 | |||
| PA7 (methylene blue) | 80 | 298 | −8.13 | 10.85 | 63.61 |
| 98 | 318 | −9.31 | |||
| 109 | 338 | −10.69 | |||
| PA6 (methyl red) | 25 | 298 | −3.15 | 12.21 | 51.52 |
| 29 | 318 | −4.17 | |||
| 32 | 338 | −5.22 | |||
| PA7 (methyl red) | 50 | 298 | −8.35 | 6.88 | 51.07 |
| 57 | 318 | −9.35 | |||
| 63 | 338 | −10.39 |
| Organic Dye | Bioadsorbent | qmax [mg/g] | References |
|---|---|---|---|
| Methylene blue | PA6 | 41 | This study |
| PA7 | 147 | This study | |
| Weeds-based biochar | 37 | [34] | |
| Microcrystalline cellulose from oil palm fronds | 13 | [35] | |
| Hydrochars from Pinus caribaea | 149 | [36] | |
| Methyl red | PA6 | 33 | This study |
| PA7 | 115 | This study | |
| Bark of Hopbush | 37 | [37] | |
| White potato peel powder | 31 | [38] | |
| Coffee residues | 77 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolski, R.; Bazan-Wozniak, A.; Pietrzak, R. Adsorption of Methyl Red and Methylene Blue on Carbon Bioadsorbents Obtained from Biogas Plant Waste Materials. Molecules 2023, 28, 6712. https://doi.org/10.3390/molecules28186712
Wolski R, Bazan-Wozniak A, Pietrzak R. Adsorption of Methyl Red and Methylene Blue on Carbon Bioadsorbents Obtained from Biogas Plant Waste Materials. Molecules. 2023; 28(18):6712. https://doi.org/10.3390/molecules28186712
Chicago/Turabian StyleWolski, Robert, Aleksandra Bazan-Wozniak, and Robert Pietrzak. 2023. "Adsorption of Methyl Red and Methylene Blue on Carbon Bioadsorbents Obtained from Biogas Plant Waste Materials" Molecules 28, no. 18: 6712. https://doi.org/10.3390/molecules28186712
APA StyleWolski, R., Bazan-Wozniak, A., & Pietrzak, R. (2023). Adsorption of Methyl Red and Methylene Blue on Carbon Bioadsorbents Obtained from Biogas Plant Waste Materials. Molecules, 28(18), 6712. https://doi.org/10.3390/molecules28186712