Antioxidant and Hypolipidemic Activities of Cinnamic Acid Derivatives
Abstract
:1. Introduction
2. Results and Discussion
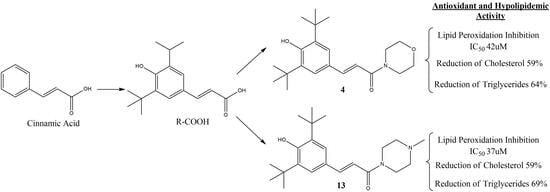
2.1. Synthesis
- DMAP: 4-dimethylaminopyridine; DCC: N,N’-dicyclohexylcarbodiimide; THF: tetrahydrofuran;
- I: 3-(4-hydroxy-3-methoxyphenyl)acrylic acid; II: 3-(4-hydroxy-3,5-dimethoxyphenyl)acrylic acid;
- III: 3-(3,4-dimethoxyphenyl)acrylic acid; IV: 3-(4-hydroxyphenyl)acrylic acid;
- V: 3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylic acid;
- compound 1: (E)-3-(4-hydroxy-3-methoxyphenyl)-1-morpholinoprop-2-en-1-one;
- compound 2: (E)-3-(4-hydroxy-3,5-dimethoxyphenyl)-1-morpholinoprop-2-en-1-one;
- compound 3: (E)-3-(3,4-dimethoxyphenyl)-1-morpholinoprop-2-en-1-one;
- compound 4: (E,Z)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-morpholinoprop-2-en-1-one;
- compound 5: (E)-pyridin-3-ylmethyl 3-(4-hydroxy-3-methoxyphenyl)acrylate;
- compound 6: (E)-pyridin-3-ylmethyl 3-(4-hydroxy-3,5-dimethoxyphenyl)acrylate;
- compound 7: (E)-pyridin-3-ylmethyl 3-(3,4-dimethoxyphenyl)acrylate;
- compound 8: (E)-pyridin-3-ylmethyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylate;
- compound 9: (E)-1-(3-(3,4-dimethoxyphenyl)acryloyl)piperidin-4-one;
- compound 10: (E)-1-(3-(3,5-di-tert-butyl-4-hydroxyphenyl)acryloyl)piperidin-4-one;
- compound 11: (E)-3-(4-hydroxyphenyl)-1-(4-methylpiperazin-1-yl)prop-2-en-1-one;
- compound 12: (E)-3-(3,4-dimethoxyphenyl)-1-(4-methylpiperazin-1-yl)prop-2-en-1-one;
- compound 13: (E)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-1-(4-methylpiperazin-1-yl)prop-2-en-1-one
2.2. Biological Evaluation
2.2.1. Effect on Lipid Peroxidation
2.2.2. Interaction with DPPH
2.2.3. Effect of Compounds on Hyperlipidemia in Rats
3. Materials and Methods
3.1. General Methods
3.2. Synthesis
3.2.1. General Method for the Synthesis of Compounds 1–4
3.2.2. General Method for the Synthesis of Compounds 5–8
3.2.3. General Method for the Synthesis of Compounds 9–10
3.2.4. General Method for the Synthesis of Compounds 11–13
3.3. Effect on Lipid Peroxidation
3.4. Interaction with the Stable Radical 1,1-Diphenyl-2-picrylhydrazyl (DPPH)
3.5. Effect on Plasma Total Cholesterol, Triglyceride and LDL-Cholesterol Levels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sánchez, A.; Calpena, A.C.; Clares, B. Evaluating the oxidative stress in inflammation: Role of melatonin. Int. J. Mol. Sci. 2015, 16, 16981–17004. [Google Scholar] [CrossRef]
- Li, H.; Horke, S.; Förstermann, U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 2013, 34, 313–319. [Google Scholar] [CrossRef]
- Apryatin, S.A.; Sidorova, Y.S.; Shipelin, V.A.; Balakina, A.; Trusov, N.V.; Mazo, V.K. Neuromotor activity, anxiety and cognitive function in the in vivo model of alimentary hyperlipidemia and obesity. Bull. Exp. Biol. Med. 2017, 163, 37–44. [Google Scholar] [CrossRef]
- Gancheva, S.; Galunska, B.; Zhelyazkova-Savova, M. Diets rich in saturated fat and fructose induce anxiety and depression-like behaviours in the rat: Is there a role for lipid peroxidation? Int. J. Exp. Pathol. 2017, 98, 296–306. [Google Scholar] [CrossRef]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-inflammatory activities of cinnamomum cassia constituents in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2012, 2012, 429320. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Zou, Y.; Kim, A.R.; Kim, J.E.; Choi, J.S.; Chung, H.Y. Peroxynitrite scavenging activity of sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid) isolated from Brassica juncea. J. Agric. Food Chem. 2002, 50, 5884–5890. [Google Scholar] [CrossRef]
- Rychlicka, M.; Rot, A.; Gliszczyńska, A. Biological properties, health benefits and enzymatic modifications of dietary methoxylated derivatives of cinnamic acid. Foods 2021, 10, 1417. [Google Scholar] [CrossRef]
- Malik, N.; Dhiman, P. New approaches and advancements in drug development from phenolic p-coumaric acid. Curr. Top. Med. Chem. 2022, 22, 1515–1529. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Athanasekou, C.; Rekka, E.A. Dual antioxidant structures with potent anti-inflammatory, hypolipidemic and cytoprotective properties. Bioorg. Med. Chem. Lett. 2017, 27, 4800–4804. [Google Scholar] [CrossRef]
- Jin, X.L.; Wei, X.; Qi, F.M.; Yu, S.S.; Zhou, B.; Bai, S. Characterization of hydroxycinnamic acid derivatives binding to bovine serum albumin. Org. Biomol. Chem. 2012, 10, 3424–3431. [Google Scholar] [CrossRef]
- Zheng, L.F.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.L. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu(II) ions: Mechanism and structure-activity relationship. Food Chem. Toxicol. 2008, 46, 149–156. [Google Scholar] [CrossRef]
- Maistro, E.L.; Angeli, J.P.; Andrade, S.F.; Mantovani, M.S. In vitro genotoxicity assessment of caffeic, cinnamic and ferulic acids. Genet. Mol. Res. 2011, 10, 1130–1140. [Google Scholar] [CrossRef]
- Chrysselis, M.C.; Rekka, E.A.; Kourounakis, P.N. Hypocholesterolemic and hypolipidemic activity of some novel morpholine derivatives with antioxidant activity. J. Med. Chem. 2000, 43, 609–612. [Google Scholar] [CrossRef]
- Tooulia, K.K.; Theodosis-Nobelos, P.; Rekka, E.A. Thiomorpholine derivatives with hypolipidemic and antioxidant activity. Arch. Pharm. 2015, 348, 629–634. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Kourti, M.; Gavalas, A.; Rekka, E.A. Amides of non-steroidal anti-inflammatory drugs with thiomorpholine can yield hypolipidemic agents with improved anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2016, 26, 910–913. [Google Scholar] [CrossRef]
- Kim, J.H.; Shyam, P.K.; Kim, M.J.; Lee, H.J.; Lee, J.T.; Jang, H.Y. Enantioselective synthesis and antioxidant activity of 3,4,5-substituted piperidine derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3119–3121. [Google Scholar] [CrossRef]
- Pandey, P.; Chawla, P. Syntheses, characterization and biological activity of novel 2,6-disubstituted piperidine-4-one derivatives. Inter. J. Pharm. Chem. Biol. Sci. 2012, 2, 305–309. [Google Scholar]
- Mancilla, T.; Canillo, L.; Zamudio, L.S.; Beltrán, H.I.; Farán, N. Synthesis and characterization of piperazine-2,6-diones. Org. Prep. Proced. Int. 2009, 34, 87–94. [Google Scholar] [CrossRef]
- Cohen, M. Antihyperlipidemic properties of beta-pyridylcarbinol. A review of preclinical studies. Life Sci. 1985, 37, 1949–1961. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Nithiya, S.; Karthik, N.; Jayabharathi, J. In vitro antioxidant activity of hundred piperidone derivatives. Intl. J. Pharm. Pharm. Sci. 2011, 3, 254–256. [Google Scholar]
- Guitard, R.; Nardello-Rataj, V.; Aubry, J.M. Theoretical and kinetic tools for selecting effective antioxidants: Application to the protection of omega-3 oils with natural and synthetic phenols. Int. J. Mol. Sci. 2016, 17, 1220. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Antioxidant serine-(NSAID) hybrids with anti-inflammatory and hypolipidemic potency. Molecules 2021, 26, 4060. [Google Scholar] [CrossRef] [PubMed]
- Tsiakitzis, K.; Papagiouvannis, G.; Theodosis-Nobelos, P.; Tziona, P.; Kourounakis, P.N.; Rekka, E.A. Synthesis, antioxidant and anti-inflammatory effects of antioxidant acid amides with GABA and N-acyl-pyrrolidin-2-ones. Current Chem. Biol. 2017, 11, 127–139. [Google Scholar] [CrossRef]
- Tuñón, J.; Badimón, L.; Bochaton-Piallat, M.L.; Cariou, B.; Daemen, M.J.; Egido, J.; Evans, P.C.; Hoefer, I.E.; Ketelhuth, D.F.J.; Lutgens, E.; et al. Identifying the anti-inflammatory response to lipid lowering therapy: A position paper from the working group on atherosclerosis and vascular biology of the European Society of Cardiology. Cardiovasc. Res. 2019, 115, 10–19. [Google Scholar] [CrossRef]
- Zarzecki, M.S.; Araujo, S.M.; Bortolotto, V.C.; de Paula, M.T.; Jesse, C.R.; Prigol, M. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol. Rep. 2014, 1, 200–208. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Tuzikov, F.V.; Vasileva, E.D.; Cherkanova, M.S.; Tuzikova, N.A. Fractional composition of blood serum lipoproteins in mice and rats with Triton WR 1339-induced lipemia. Bull. Exp. Biol. Med. 2010, 149, 567–570. [Google Scholar] [CrossRef]
- Quispe, R.; Sweeney, T.; Varma, B.; Agarwala, A.; Michos, E.D. Recent Updates in Hypertriglyceridemia Management for Cardiovascular Disease Prevention. Curr. Atheroscler. Rep. 2022, 24, 767–778. [Google Scholar] [CrossRef]
- Ullah, S.; Park, C.; Ikram, M.; Kang, D.; Lee, S.; Yang, J.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogues. Bioorg. Chem. 2019, 87, 43–55. [Google Scholar] [CrossRef]
- Correa, E.A.; Högestätt, E.D.; Sterner, O.; Echeverri, F.; Zygmunt, P.M. In vitro TRPV1 activity of piperine derived amides. Bioorg. Med. Chem. 2010, 18, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.; Liu, X.; Fang, Q.; Wang, Z.; Fu, L.; Liu, Z.; Wang, Y.; Zhao, Y.; Li, X.; et al. Discovery of a new inhibitor of myeloid differentiation 2 from cinnamamide derivatives with anti-inflammatory activity in sepsis and acute lung injury. J. Med. Chem. 2016, 59, 2436–2451. [Google Scholar] [CrossRef] [PubMed]
- Cerbai, G.; Turbanti, L.; Bianchini, P.; Bramanti, G.; Tellini, N. Sintesi e indagine farmacologica di una serie di derivati amidici dell’acido 3,4,5-trimetossicinnamico e analoghi [Synthesis and pharmaceutical findings on a series of amido derivatives of 3,4,5-trimethoxycinnamoyl acid and its analogs]. Boll. Chim. Farm. 1967, 106, 837–854. [Google Scholar] [PubMed]
- Fokoue, H.H.; Marques, J.V.; Correia, M.V.; Yamaguchi, L.F.; Qu, X.; Aires-de-Sousa, J.; Scotti, M.T.; Lopes, N.P.; Kato, M.J. Fragmentation pattern of amides by EI and HRESI: Study of protonation sites using DFT-3LYP data. RSC Adv. 2018, 8, 21407–21413. [Google Scholar] [CrossRef]
- Yang, X.D.; Zeng, X.H.; Zhao, Y.H.; Wang, X.Q.; Pan, Z.Q.; Li, L.; Zhang, H.B. Silica gel-mediated amide bond formation: An environmentally benign method for liquid-phase synthesis and cytotoxic activities of amides. J. Comb. Chem. 2010, 12, 307–310. [Google Scholar] [CrossRef]
- Yeşilada, A.; Zorlu, E.; Aksu, F.; Yeşilada, E. 3,4-Dimethoxy cinnamic acid tertiary amides: Synthesis and evaluation of antiinflammatory and analgesic activities. Farmaco 1996, 51, 595–599. [Google Scholar]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. Ferulic, sinapic, 3,4-dimethoxycinnamic acid and indomethacin derivatives with antioxidant, anti-inflammatory and hypolipidemic functionality. Antioxidants 2023, 12, 1436. [Google Scholar] [CrossRef]


| Compound | Lipid Peroxidation Inhibition: IC50 (μΜ) a |
|---|---|
| 4 | 42 |
| 5 | 545 |
| 6 | 189 |
| 8 | 42 |
| 9 | 205 |
| 10 | 37 |
| 13 | 41 |
| Trolox | 25 |
| Compound | Percent Interaction | IC50 (μΜ) b | |||
|---|---|---|---|---|---|
| 200 μΜ | 100 μΜ | 50 μΜ | 25 μΜ | ||
| 2 | 90.70 | 80.68 | 74.19 | 34.74 | 31.83 |
| 4 | 95.63 | 81.88 | 78.20 | 32.92 | 31.75 |
| 5 | 65.00 | 62.27 | 27.16 | 0.00 | 99.66 |
| 6 | 71.93 | 69.56 | 27.56 | 0.00 | 87.07 |
| 8 | 95.08 | 81.41 | 70.11 | 34.01 | 34.48 |
| 10 | 90.48 | 82.50 | 69.38 | 33.65 | 34.51 |
| 13 | 92.96 | 86.15 | 73.57 | 32.48 | 33.05 |
| Trolox | 98.00 | 92.00 | 38.00 | 22.00 | 52.01 |
| Compound | % Reduction | clogP c | |
|---|---|---|---|
| TC a | TG b | ||
| 1 | 8.4 ns | 8.4 ns | 0.95 |
| 2 | 34.1 *** | 33.0 *** | 0.73 |
| 3 | 39.9 *** | 44.6 *** | 1.42 |
| 4 | 58.6 *** | 64.2 *** | 4.55 |
| 5 | 35.1 ** | 44.6 *** | 1.92 |
| 6 | 50.8 *** | 69.5 *** | 1.70 |
| 7 | 33.0 *** | 35.1 *** | 2.39 |
| 8 | 40.2 * | 35.1 *** | 5.52 |
| 9 | 38.9 *** | 24.6 * | 1.36 |
| 10 | 51.1 *** | 35.7 * | 4.49 |
| 11 | 64.3 *** | 51.5 *** | 1.66 |
| 12 | 59.8 *** | 61.9 *** | 1.86 |
| 13 | 59.3 *** | 69.4 *** | 5.11 |
| Simvastatin | 73 *** | - | 4.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouni, C.; Theodosis-Nobelos, P.; Rekka, E.A. Antioxidant and Hypolipidemic Activities of Cinnamic Acid Derivatives. Molecules 2023, 28, 6732. https://doi.org/10.3390/molecules28186732
Nouni C, Theodosis-Nobelos P, Rekka EA. Antioxidant and Hypolipidemic Activities of Cinnamic Acid Derivatives. Molecules. 2023; 28(18):6732. https://doi.org/10.3390/molecules28186732
Chicago/Turabian StyleNouni, Christina, Panagiotis Theodosis-Nobelos, and Eleni A. Rekka. 2023. "Antioxidant and Hypolipidemic Activities of Cinnamic Acid Derivatives" Molecules 28, no. 18: 6732. https://doi.org/10.3390/molecules28186732
APA StyleNouni, C., Theodosis-Nobelos, P., & Rekka, E. A. (2023). Antioxidant and Hypolipidemic Activities of Cinnamic Acid Derivatives. Molecules, 28(18), 6732. https://doi.org/10.3390/molecules28186732