γ-Cyclodextrin Metal-Organic Frameworks: Do Solvents Make a Difference?
Abstract
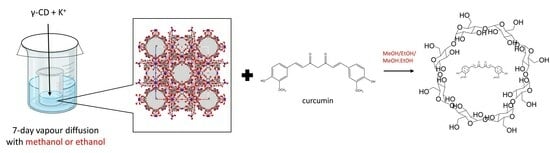
:1. Introduction
2. Results
2.1. Characterisation
2.2. Encapsulation Efficiency and Loading Capacity
3. Discussion
3.1. Characterisation
3.2. Encapsulation Efficiency and Loading Capacity
4. Materials and Methods
4.1. Materials
4.2. Synthesis of γ-CD-MOFs
4.3. Encapsulation of Curcumin in γ-CD-MOFs
4.4. Characterisation
4.4.1. Scanning Electron Microscopy (SEM)
4.4.2. Surface Area and Pore Volume Measurements
4.4.3. Fourier Transform Infrared (FT-IR) Spectroscopy
4.4.4. Powder X-ray Diffraction (PXRD)
4.5. Encapsulation Efficiency and Loading Capacity
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islamoglu, T.; Goswami, S.; Li, Z.; Howarth, A.J.; Farha, O.K.; Hupp, J.T. Postsynthetic Tuning of Metal–Organic Frameworks for Targeted Applications. Acc. Chem. Res. 2017, 50, 805–813. [Google Scholar] [CrossRef]
- Tibbetts, I.; Kostakis, G.E. Recent Bio-Advances in Metal-Organic Frameworks. Molecules 2020, 25, 1291. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, G.; Cleary, B.M.; Morrell, C.T.; Durbin, C.G.; Brinks, A.L.; Tietjen, J.; Mirica, K.A. CD-MOF-1 for CO2 Uptake: Remote and Hybrid Green Chemistry Synthesis of a Framework Material with Environmentally Conscious Applications. J. Chem. Educ. 2023, 100, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Xu, C.; Fang, R.; Luque, R.; Chen, L.; Li, Y. Functional Metal–Organic Frameworks for Catalytic Applications. Coord. Chem. Rev. 2019, 388, 268–292. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Lau, J.; Fan, W.; Li, Q.; Zhang, C.; Huang, P.; Chen, X. Recent Progress in Nanoscale Metal-Organic Frameworks for Drug Release and Cancer Therapy. Nanomedicine 2019, 14, 1343–1365. [Google Scholar] [CrossRef]
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal–Organic Frameworks and Their Applications. Acc. Chem. Res. 2021, 54, 1440–1453. [Google Scholar] [CrossRef]
- Shen, M.; Liu, D.; Ding, T. Cyclodextrin-Metal-Organic Frameworks (CD-MOFs): Main Aspects and Perspectives in Food Applications. Curr. Opin. Food Sci. 2021, 41, 8–15. [Google Scholar] [CrossRef]
- Smaldone, R.A.; Forgan, R.S.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.Z.; Yaghi, O.M.; Stoddart, J.F. Metal–Organic Frameworks from Edible Natural Products. Angew. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef]
- Moussa, Z.; Hmadeh, M.; Abiad, M.G.; Dib, O.H.; Patra, D. Encapsulation of Curcumin in Cyclodextrin-Metal Organic Frameworks: Dissociation of Loaded CD-MOFs Enhances Stability of Curcumin. Food Chem. 2016, 212, 485–494. [Google Scholar] [CrossRef]
- Chen, Y.; Tai, K.; Ma, P.; Su, J.; Dong, W.; Gao, Y.; Mao, L.; Liu, J.; Yuan, F. Novel γ-Cyclodextrin-Metal–Organic Frameworks for Encapsulation of Curcumin with Improved Loading Capacity, Physicochemical Stability and Controlled Release Properties. Food Chem. 2021, 347, 128978. [Google Scholar] [CrossRef]
- Sun, Q.; Sheng, J.; Yang, R. Encapsulation of Curcumin in CD-MOFs: Promoting Its Incorporation into Water-Based Products and Consumption. Food Funct. 2021, 12, 10795–10805. [Google Scholar] [CrossRef]
- Qiu, C.; McClements, D.J.; Jin, Z.; Wang, C.; Qin, Y.; Xu, X.; Wang, J. Development of Nanoscale Bioactive Delivery Systems Using Sonication: Glycyrrhizic Acid-Loaded Cyclodextrin Metal-Organic Frameworks. J. Colloid Interface Sci. 2019, 553, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Julian McClements, D.; Jin, Z.; Qin, Y.; Hu, Y.; Xu, X.; Wang, J. Resveratrol-Loaded Core-Shell Nanostructured Delivery Systems: Cyclodextrin-Based Metal-Organic Nanocapsules Prepared by Ionic Gelation. Food Chem. 2020, 317, 126328. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, L.; Guo, T.; Zhang, G.; Wang, C.; Li, H.; Li, X.; Singh, V.; Chen, W.; Gref, R.; et al. A “Ship-in-a-Bottle” Strategy to Create Folic Acid Nanoclusters inside the Nanocages of γ-Cyclodextrin Metal-Organic Frameworks. Int. J. Pharm. 2019, 556, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, F.; Du, M.; Xie, C.; Xie, X.; Zhang, H.; Meng, X.; Li, A.; Deng, T. Encapsulation of Catechin into Nano-Cyclodextrin-Metal-Organic Frameworks: Preparation, Characterization, and Evaluation of Storage Stability and Bioavailability. Food Chem. 2022, 394, 133553. [Google Scholar] [CrossRef]
- Lu, W.; Guo, J.; Zhou, J.; Ke, L.; Liu, S.; Gao, G.; Wang, H.; Ding, W.; Rao, P. Hypothesis Review: The Direct Interaction of Food Nanoparticles with the Lymphatic System. Food Sci. Hum. Wellness 2012, 1, 61–64. [Google Scholar] [CrossRef]
- Sadeh, P.; Zeinali, S.; Rastegari, B.; Najafipour, I. Size Optimization of Mesoporous β-Cyclodextrin Metal-Organic Frameworks as Bio-MOFs. J. Cryst. Growth 2023, 620, 127348. [Google Scholar] [CrossRef]
- He, Y.; Hou, X.; Guo, J.; He, Z.; Guo, T.; Liu, Y.; Zhang, Y.; Zhang, J.; Feng, N. Activation of a Gamma–Cyclodextrin–Based Metal–Organic Framework Using Supercritical Carbon Dioxide for High–Efficient Delivery of Honokiol. Carbohydr. Polym. 2020, 235, 115935. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Xu, X.; Li, X.; Lv, N.; Singh, V.; Stoddart, J.F.; York, P.; Xu, X.; Gref, R.; et al. Optimized Synthesis and Crystalline Stability of γ-Cyclodextrin Metal-Organic Frameworks for Drug Adsorption. Int. J. Pharm. 2016, 514, 212–219. [Google Scholar] [CrossRef]
- Furukawa, Y.; Ishiwata, T.; Sugikawa, K.; Kokado, K.; Sada, K. Nano- and Microsized Cubic Gel Particles from Cyclodextrin Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2012, 51, 10566–10569. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; McGillivray, D.J. Recent Advances to Improve Curcumin Oral Bioavailability. Trends Food Sci. Technol. 2021, 110, 253–266. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent Advances in Emulsion-Based Delivery Approaches for Curcumin: From Encapsulation to Bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Kotra, V.S.R.; Satyabanta, L.; Goswami, T.K. A Critical Review of Analytical Methods for Determination of Curcuminoids in Turmeric. J. Food Sci. Technol. 2019, 56, 5153–5166. [Google Scholar] [CrossRef]
- Pan, X.; Junejo, S.A.; Tan, C.P.; Zhang, B.; Fu, X.; Huang, Q. Effect of Potassium Salts on the Structure of γ-Cyclodextrin MOF and the Encapsulation Properties with Thymol. J. Sci. Food Agric. 2022, 102, 6387–6396. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.Y.; Kadota, K.; Nakajima, T.; Uchiyama, H.; Tanaka, S.; Tozuka, Y. Crystalline Rearranged CD-MOF Particles Obtained via Spray-Drying Synthesis Applied to Inhalable Formulations with High Drug Loading. Cryst. Growth Des. 2022, 22, 1143–1154. [Google Scholar] [CrossRef]
- Kathuria, A.; Pauwels, A.-K.; Buntinx, M.; Shin, J.; Harding, T. Inclusion of Ethanol in a Nano-Porous, Bio-Based Metal Organic Framework. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 91–98. [Google Scholar] [CrossRef]
- Shen, M.; Forghani, F.; Kong, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Antibacterial Applications of Metal–Organic Frameworks and Their Composites. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1397–1419. [Google Scholar] [CrossRef]
- Singh, V.; Guo, T.; Xu, H.; Wu, L.; Gu, J.; Wu, C.; Gref, R.; Zhang, J. Moisture Resistant and Biofriendly CD-MOF Nanoparticles Obtained via Cholesterol Shielding. Chem. Commun. 2017, 53, 9246–9249. [Google Scholar] [CrossRef]
- Smilgies, D.-M. Scherrer Grain-Size Analysis Adapted to Grazing-Incidence Scattering with Area Detectors. Erratum. J. Appl. Crystallogr. 2009, 42, 1030–1034. [Google Scholar] [CrossRef]
- Rajkumar, T.; Kukkar, D.; Kim, K.-H.; Sohn, J.R.; Deep, A. Cyclodextrin-Metal–Organic Framework (CD-MOF): From Synthesis to Applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Lv, N.; Guo, T.; Liu, B.; Wang, C.; Singh, V.; Xu, X.; Li, X.; Chen, D.; Gref, R.; Zhang, J. Improvement in Thermal Stability of Sucralose by γ-Cyclodextrin Metal-Organic Frameworks. Pharm. Res. 2017, 34, 269–278. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, J. In Situ Growth of Cyclodextrin-Based Metal Organic Framework Air Filters for Reusable SO2 Adsorbent Applications. Macromol. Mater. Eng. 2023, 308, 2200645. [Google Scholar] [CrossRef]
- Parambil, J.V.; Poornachary, S.K.; Tan, R.B.H.; Heng, J.Y.Y. Influence of Solvent Polarity and Supersaturation on Template-Induced Nucleation of Carbamazepine Crystal Polymorphs. J. Cryst. Growth 2017, 469, 84–90. [Google Scholar] [CrossRef]
- Neugebauer, P.; Cardona, J.; Besenhard, M.O.; Peter, A.; Gruber-Woelfler, H.; Tachtatzis, C.; Cleary, A.; Andonovic, I.; Sefcik, J.; Khinast, J.G. Crystal Shape Modification via Cycles of Growth and Dissolution in a Tubular Crystallizer. Cryst. Growth Des. 2018, 18, 4403–4415. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, T.; Zhang, K.; Chen, J.; Wang, C.; Ren, X.; Wang, Q.; Yang, Y.; Liu, C.; Tan, W.; et al. Simultaneous Improvement to Solubility and Bioavailability of Active Natural Compound Isosteviol Using Cyclodextrin Metal-Organic Frameworks. Acta Pharm. Sin. B 2021, 11, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.P.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T. Supercritical Processing as a Route to High Internal Surface Areas and Permanent Microporosity in Metal−Organic Framework Materials. J. Am. Chem. Soc. 2009, 131, 458–460. [Google Scholar] [CrossRef]
- Matsuyama, K.; Hayashi, N.; Yokomizo, M.; Kato, T.; Ohara, K.; Okuyama, T. Supercritical Carbon Dioxide-Assisted Drug Loading and Release from Biocompatible Porous Metal–Organic Frameworks. J. Mater. Chem. B 2014, 2, 7551–7558. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Nanri, A.; Murata, I.; Kanamoto, I. Characterization of Inclusion Complex of Coenzyme Q10 with the New Carrier CD-MOF-1 Prepared by Solvent Evaporation. AAPS PharmSciTech 2018, 19, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Natarajan, R.K.; Natarajan, S.; Rathikha, R. Structural Investigation on Curcumin. Asian J. Chem. 2008, 20, 2903–2913. [Google Scholar]
- Zhang, W.; Guo, T.; Wang, C.; He, Y.; Zhang, X.; Li, G.; Chen, Y.; Li, J.; Lin, Y.; Xu, X.; et al. MOF Capacitates Cyclodextrin to Mega-Load Mode for High-Efficient Delivery of Valsartan. Pharm. Res. 2019, 36, 117. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Jiang, Y.; Zhou, F.; Wu, Y.; Jiang, H.; Wang, R.; Xu, Q.; Hua, C. Encapsulating Quercetin in Cyclodextrin Metal–Organic Frameworks Improved Its Solubility and Bioavailability. J. Sci. Food Agric. 2022, 102, 3887–3896. [Google Scholar] [CrossRef]
- He, W.; Ye, K.; Li, H.; Wang, C.; Wei, H.; Dang, L. Novel γ-Cyclodextrin-Based Metal–Organic Frameworks for the Effective Encapsulation of Oregano Essential Oil and Controlled Release. New J. Chem. 2023, 47, 10322–10332. [Google Scholar] [CrossRef]
- Guo, Q.; Su, J.; Xie, W.; Tu, X.; Yuan, F.; Mao, L.; Gao, Y. Curcumin-Loaded Pea Protein Isolate-High Methoxyl Pectin Complexes Induced by Calcium Ions: Characterization, Stability and in Vitro Digestibility. Food Hydrocoll. 2020, 98, 105284. [Google Scholar] [CrossRef]
- Yang, A.; Liu, H.; Li, Z.; Li, L.; Li, W.; Liu, K. Green Synthesis of β-Cyclodextrin Metal–Organic Frameworks and the Adsorption of Quercetin and Emodin. Polyhedron 2019, 159, 116–126. [Google Scholar] [CrossRef]
- Chen, Y.; Su, J.; Dong, W.; Xu, D.; Cheng, L.; Mao, L.; Gao, Y.; Yuan, F. Cyclodextrin-Based Metal–Organic Framework Nanoparticles as Superior Carriers for Curcumin: Study of Encapsulation Mechanism, Solubility, Release Kinetics, and Antioxidative Stability. Food Chem. 2022, 383, 132605. [Google Scholar] [CrossRef]
- Cui, Z.; Yao, L.; Ye, J.; Wang, Z.; Hu, Y. Solubility Measurement and Thermodynamic Modelling of Curcumin in Twelve Pure Solvents and Three Binary Solvents at Different Temperature (T = 278.15–323.15 K). J. Mol. Liq. 2021, 338, 116795. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]






| Samples | BET Surface Area (m2/g) | Langmuir Surface Area (m2/g) | Pore Diameters (Å) | Crystallite Size (nm) |
|---|---|---|---|---|
| CD-MOF (M) | 799.4 ± 14.6 a | 813.7 ± 14.3 a | 18.6 ± 0.1 a | 398 ± 38 |
| CD-MOF (E) | 719.1 ± 13.4 a | 848.1 ± 10.2 a | 18.7 ± 0.2 a | 750 ± 56 |
| Cur-CD-MOF (M, m) | 1.6 ± 0.4 b | - | - | 372 ± 76 |
| Cur-CD-MOF (M, e) | 1.3 ± 0.2 b | - | - | - |
| Cur-CD-MOF (E, m) | 1.7 ± 0.6 b | - | - | - |
| Cur-CD-MOF (E, e) | 1.6 ± 0.5 b | - | - | 270 ± 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.X.; Murray, B.S.; Mackie, A.R.; Ettelaie, R.; Sadeghpour, A.; Frison, R. γ-Cyclodextrin Metal-Organic Frameworks: Do Solvents Make a Difference? Molecules 2023, 28, 6876. https://doi.org/10.3390/molecules28196876
Oh JX, Murray BS, Mackie AR, Ettelaie R, Sadeghpour A, Frison R. γ-Cyclodextrin Metal-Organic Frameworks: Do Solvents Make a Difference? Molecules. 2023; 28(19):6876. https://doi.org/10.3390/molecules28196876
Chicago/Turabian StyleOh, Jia X., Brent S. Murray, Alan R. Mackie, Rammile Ettelaie, Amin Sadeghpour, and Ruggero Frison. 2023. "γ-Cyclodextrin Metal-Organic Frameworks: Do Solvents Make a Difference?" Molecules 28, no. 19: 6876. https://doi.org/10.3390/molecules28196876
APA StyleOh, J. X., Murray, B. S., Mackie, A. R., Ettelaie, R., Sadeghpour, A., & Frison, R. (2023). γ-Cyclodextrin Metal-Organic Frameworks: Do Solvents Make a Difference? Molecules, 28(19), 6876. https://doi.org/10.3390/molecules28196876