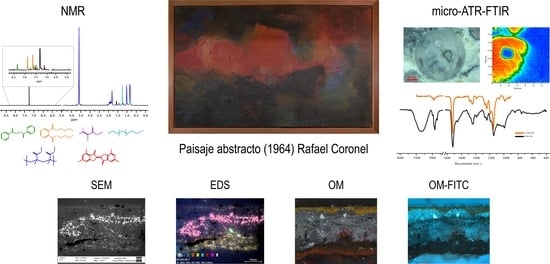
Microanalytical Characterization of an Innovative Modern Mural Painting Technique by SEM-EDS, NMR and Micro-ATR-FTIR among Others
Abstract
:1. Introduction
2. Results
2.1. Description of the Technique and Observations of the Conservation State
2.2. Optical Microscopy Study
2.3. Elemental Composition Analysis by SEM-EDS
2.4. Organic Compound Elucidation by NMR
2.4.1. Binding Medium
2.4.2. Monomer
2.4.3. Catalyst
2.4.4. Plasticizer
2.4.5. Additive
2.4.6. Pigment
2.5. ATR-FTIR Study
2.5.1. pMMA
2.5.2. Organic Pigment
2.5.3. Inorganic Pigment
2.6. Micro-ATR-FTIR Analysis
2.7. GC/MS Analysis
3. Discussion
4. Material and Methods
4.1. Sampling
4.2. Optical Microscopy
4.3. Scanning Electron Microscopy—Energy Dispersive X-ray Spectroscopy
4.4. Nuclear Magnetic Resonance Spectroscopy
4.5. Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy
4.6. Micro-Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy
4.7. Gas Chromatography/Mass Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Coffey, M.K. How a Revolutionary Art Became Official Culture; Duke University Press: Durham, NC, USA, 2012; ISBN 9780822394273. [Google Scholar]
- Rita Eder Variations. Painting in Mexico during the Fifties and Sixties. In Defyng Stability, Artistic Processes in Mexico, 1952–1967; Eder, R., Ed.; UNAM: Ciudad de México, México, 2014; pp. 145–163. ISBN 978-84-15832-39-3. [Google Scholar]
- Shifra, M. Goldman Contemporary Mexican Painting in a Time of Change; University of Texas Press: Austin, TX, USA, 1981; ISBN 0292710615. [Google Scholar]
- Spyros, A.; Anglos, D. Study of Aging in Oil Paintings by 1D and 2D NMR Spectroscopy. Anal. Chem. 2004, 76, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Tortora, M.; Sfarra, S.; Chiarini, M.; Daniele, V.; Taglieri, G.; Cerichelli, G. Non-Destructive and Micro-Invasive Testing Techniques for Characterizing Materials, Structures and Restoration Problems in Mural Paintings. Appl. Surf. Sci. 2016, 387, 971–985. [Google Scholar] [CrossRef]
- Mejía-González, A.; Jáidar, Y.; Zetina, S.; Aguilar-Rodríguez, P.; Ruvalcaba-Sil, J.L.; Esturau-Escofet, N. NMR and Other Molecular and Elemental Spectroscopies for the Characterization of Samples from an Outdoor Mural Painting by Siqueiros. Spectrochim. Acta Mol. Biomol. Spectrosc. 2022, 274, 121073. [Google Scholar] [CrossRef] [PubMed]
- Saladino, M.L.; Ridolfi, S.; Carocci, I.; Martino, D.C.; Lombardo, R.; Spinella, A.; Traina, G.; Caponetti, E. A Multi-Analytical Non-Invasive and Micro-Invasive Approach to Canvas Oil Paintings. General Considerations from a Specific Case. Microchem. J. 2017, 133, 133–607. [Google Scholar] [CrossRef]
- Tanasi, D.; Greco, E.; di Tullio, V.; Capitani, D.; Gullì, D.; Ciliberto, E. 1H-1H NMR 2D-TOCSY, ATR FT-IR and SEM-EDX for the Identification of Organic Residues on Sicilian Prehistoric Pottery. Microchem. J. 2017, 135, 140–147. [Google Scholar] [CrossRef]
- Colantonio, C.; Baldassarri, P.; Avino, P.; Astolfi, M.L.; Visco, G. Visual and Physical Degradation of the Black and White Mosaic of a Roman Domus under Palazzo Valentini in Rome: A Preliminary Study. Molecules 2022, 27, 7765. [Google Scholar] [CrossRef] [PubMed]
- Lizeth, M.-D.A.; Gilda, S.-M.; Laura, F.; Alejandro, M.; María, Á.-J.S.; Alejandra, C.-A.L.; Ivette, N.-R.L.; de Rafael Coronel, S.-C.A.P.A. Informe de Los Trabajos de Conservación y Restauración Realizados en la Práctica Intersemestral del 17 Junio al 14 de Julio Del 2013; Instituto Nacional de Antropología e Historia: Mexico City, Mexico, 2013; Available online: https://mediateca.inah.gob.mx/repositorio/islandora/object/informe%3A581 (accessed on 3 October 2022).
- White, A.J.; Filisko, F.E. Tacticity Determination of Poly(Methyl Methacrylate) (PMMA) by High-Resolution NMR. J. Polym. Sci. Polym. Lett. Ed. 1982, 20, 525–529. [Google Scholar] [CrossRef]
- Lipschitz, I. The Vibrational Spectrum of Poly(Methyl Methacrylate): A Review. Polym. Plast. Technol. Eng. 1982, 19, 53–106. [Google Scholar] [CrossRef]
- Willis, H.A.; Zichy, V.J.I.; Hendra, P.J. The Laser-Raman and Infra-Red Spectra of Poly(Methyl Methacrylate). Polymer 1969, 10, 737–746. [Google Scholar] [CrossRef]
- Price, B.A.; Pretzel, B. Suzanne Quillen Lomax Infrared and Raman Users Group Spectral Database. Available online: www.irug.org (accessed on 22 October 2022).
- Sakthidharan, C.P.; Niewa, R.; Zherebtsov, D.A.; Podgornov, F.V.; Matveychuk, Y.V.; Bartashevich, E.V.; Nayfert, S.A.; Adonin, S.A.; Gavrilyak, M.V.; Boronin, V.A.; et al. Crystal Structures and Dielectric Properties of 4,4′-Dimethyl-6,6′-Dichlorothioindigo (Pigment Red 181). Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2021, 77, 23–30. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, Y.; Sun, S. Influence of Eu Doping on the Microstructure and Photoluminescence of CdS Nanocrystals. Appl. Surf. Sci. 2012, 258, 7658–7663. [Google Scholar] [CrossRef]
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR Spectral Collection of Conservation Materials in the Extended Region of 4000–4080 cm−1. Anal. Bioanal. Chem. 2016, 408, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Thambidurai, M.; Murugan, N.; Muthukumarasamy, N.; Agilan, S.; Vasantha, S.; Balasundaraprabhu, R. Influence of the Cd/S Molar Ratio on the Optical and Structural Properties of Nanocrystalline CdS Thin Films. J. Mater. Sci. Technol. 2010, 26, 193–199. [Google Scholar] [CrossRef]
- Sobhana, S.S.L.; Vimala Devi, M.; Sastry, T.P.; Mandal, A.B. CdS Quantum Dots for Measurement of the Size-Dependent Optical Properties of Thiol Capping. J. Nanoparticle Res. 2011, 13, 1747–1757. [Google Scholar] [CrossRef]
- Kulzer & Co., GmbH. Verfahren Zür Herstellung von Prothesen Für Zarnarztliche Order Andere Zweck Aus Polymerisierten Organischen Verbindungen. DE737058C. 1936. Available online: https://worldwide.espacenet.com/patent/search/family/025760434/publication/DE737058C?q=pn%3DDE737058C (accessed on 25 October 2022).
- Bieleman, J. (Ed.) Additives for Coatings; Wiley: Hoboken, NJ, USA, 2000; ISBN 9783527297856. [Google Scholar]
- BAYER AG—FARBENFABRIKEN BAYER. Improvements in or Relating to Material for Surgical or Dental Prostheses and for Dental Repairs. GB714652A. 1952. Available online: https://worldwide.espacenet.com/patent/search/family/006121592/publication/GB714652A?q=pn%3DGB714652A (accessed on 26 October 2022).
- Samad, H.A.; Jaafar, M. Effect of Polymethyl Methacrylate (PMMA) Powder to Liquid Monomer (P/L) Ratio and Powder Molecular Weight on the Properties of PMMA Cement. Polym. Plast Technol. Eng. 2009, 48, 554–560. [Google Scholar] [CrossRef]
- Silikas, N.; Al-Kheraif, A.; Watts, D.C. Influence of P/L Ratio and Peroxide/Amine Concentrations on Shrinkage-Strain Kinetics during Setting of PMMA/MMA Biomaterial Formulations. Biomaterials 2005, 26, 197–204. [Google Scholar] [CrossRef]
- Jasper, L.E.; Deramond, H.; Mathis, J.M.; Belkoff, S.M. The Effect of Monomer-to-Powder Ratio on the Material Properties of Cranioplastic. Bone 1999, 25, 27S–29S. [Google Scholar] [CrossRef] [PubMed]
- Belkoff, S.M.; Sanders, J.C.; Jasper, L.E. The Effect of the Monomer-to-Powder Ratio on the Material Properties of Acrylic Bone Cement. J. Biomed. Mater. Res. 2002, 63, 396–399. [Google Scholar] [CrossRef]




| Compound/Structure/Assignment/Correlation | Label | δH/ppm (Multiplicity 1, J/Hz) | δC/ppm | HMBC (H→C) |
|---|---|---|---|---|
poly(methyl methacrylate) | 1 | 0.85 (bs) | 16.66 | C-2, 3, 4 |
| 1 | 1.01 (bs) | 18.82 | C-2, 3, 4 | |
| 1 | 1.21 (bs) | 21.24 | C-2, 3, 4 | |
| 2 | 1.81 (bs) | 51.24–54.48 | C-1, 2, 3, 4 | |
| 3 | --- | 44.74 | - | |
| 4 | --- | 176.26–177.96 178.44 | - | |
| 5 | 3.60 (bs) | 51.93 | C-4 | |
Methyl methacrylate | 1 | 3.75 (s) | 51.93 | C-2 |
| 2 | --- | 167.86 | - | |
| 3 | --- | 136.38 | - | |
| 4 | 1.94 (s) | 18.49 | C-2, 3, 5 | |
| 5′ | 5.56 (s) | 125.61 | - | |
| 5″ | 6.10 (s) | 125.61 | C-2, 4 | |
Benzoyl peroxide | 1 | 7.60 (t, 7.49) | 133.69 | C-3 |
| 2 | 7.47 (t, 7.81) | 128.63 | C-2, 3 | |
| 3 | 8.08 (d, 6.78) | 130.28 | C-1, 3, 5 | |
| 4 | --- | 125.60 | - | |
| 5 | --- | 167.79 | - | |
Dibutyl phthalate | 1 | 7.53 (dd, 5.8, 3.4) | 131.03 | C-2, 3 |
| 2 | 7.71 (dd, 5.8, 3.4) | 128.92 | C-1, 3, 4 | |
| 3 | --- | 132.63 | - | |
| 4 | --- | 167.91 | - | |
| 5 | 4.31 (t, 6.7) | 65.72 | C-4, 6, 7 | |
| 6 | 1.72 (q, 6.9) | 30.74 | C-5, 7, 8 | |
| 7 | 1.44 (h, 7.4) | 19.28 | C-5, 6, 8 | |
| 8 | 0.96 (t, 7.4) | 13.87 | C-6, 7 | |
Linear chain alcohol  | 1 | 0.88 (t, 7.0) | 14.2 | C-2, 3 |
| 2 | 1.29 (bs) | 22.80 | C-1, 3 | |
| 3 | 1.25 (bs) | 32.10 | C-4 | |
| 4 | 1.25 (bs) | 29.40 | C-3 | |
| 5 | 1.34 (bs) | 25.80 | C-4 | |
| 6 | 1.56 (bs) | 32.89 | C-4, 5, 7 | |
| 7 | 3.64 (t, 6.7) | 63.27 | C-5, 6 | |
PR181 | 1 | --- | NA 2 | - |
| 2 | NA 2 | |||
| 3 | --- | 125.09 | - | |
| 4 | --- | 143.63 | - | |
| 5 | 7.09 (s) | 129.05 | C-3, 7, 9 | |
| 6 | --- | --- | - | |
| 7 | 7.36 | 121.98 | C-3, 5 | |
| 8 | --- | NA 2 | - | |
| 9 | 2.73 | 18.85 | C-3, 4, 5 |
| Component | Wavenumber (cm−1)/[Intensity 1]/Assignment | References |
|---|---|---|
| poly(methyl methacrylate) | 749 [m] ν(C-C) skeletal mode, 842 [m] γ(CH2), 967 [sh] γ(α-CH3), 988 [m] γ(O-CH3), 1063 [m] ν(C-C) skeletal mode, 1144 [vs] and 1190 [vs] νa(C-O-C-), 1240 [s] and 1270 [s] ν(C-O), 1386 [m] δs(C-H) of α-CH3, 1434 [s] δs(C-H) of O-CH3, 1446 [s] δa(C-H) of α-CH3, 1482 [m] δ(CH2), 1722 [vs] ν(C=O), 2847 [vw] and 2922 [sh] combination band involving O-CH3 and CH2, 2948 [m] νs(C-H) of O-CH3 with νs(C-H) of α-CH3 and νa(C-H), 2994 [m] νa(C-H) of O-CH3 and νa(C-H) of α-CH3. | [11,12] |
| Red Pigment PR 181 | 1654 [vs], 1562 [m], 1434 [s], 1386 [w], 1294 [sh], 1240 [w], 1190 [sh], 1096 [sh], 1048 [sh], 842 [vw], 823 [vw], 781 [vw], 468 [vw]. | [13,14] |
| Cadmium sulfide (CdS) | 1114 [sh], 700 [vw], 669 [vw], 607 [vw], 572 [vw] | [15,16,17,18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Rodríguez, P.; Zetina, S.; Mejía-González, A.; Esturau-Escofet, N. Microanalytical Characterization of an Innovative Modern Mural Painting Technique by SEM-EDS, NMR and Micro-ATR-FTIR among Others. Molecules 2023, 28, 564. https://doi.org/10.3390/molecules28020564
Aguilar-Rodríguez P, Zetina S, Mejía-González A, Esturau-Escofet N. Microanalytical Characterization of an Innovative Modern Mural Painting Technique by SEM-EDS, NMR and Micro-ATR-FTIR among Others. Molecules. 2023; 28(2):564. https://doi.org/10.3390/molecules28020564
Chicago/Turabian StyleAguilar-Rodríguez, Pablo, Sandra Zetina, Adrián Mejía-González, and Nuria Esturau-Escofet. 2023. "Microanalytical Characterization of an Innovative Modern Mural Painting Technique by SEM-EDS, NMR and Micro-ATR-FTIR among Others" Molecules 28, no. 2: 564. https://doi.org/10.3390/molecules28020564
APA StyleAguilar-Rodríguez, P., Zetina, S., Mejía-González, A., & Esturau-Escofet, N. (2023). Microanalytical Characterization of an Innovative Modern Mural Painting Technique by SEM-EDS, NMR and Micro-ATR-FTIR among Others. Molecules, 28(2), 564. https://doi.org/10.3390/molecules28020564