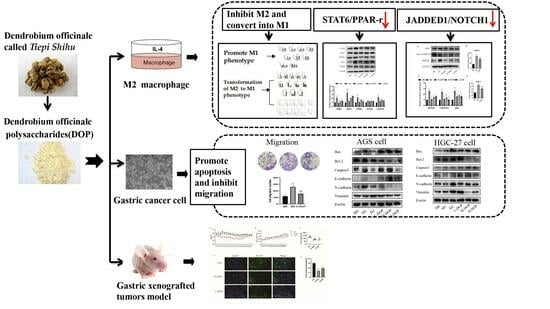
Dendrobium officinale polysaccharide Converts M2 into M1 Subtype Macrophage Polarization via the STAT6/PPAR-r and JAGGED1/NOTCH1 Signaling Pathways to Inhibit Gastric Cancer
Abstract
:1. Introduction
2. Results
2.1. Effect of DOP and Macrophage-Conditioned Medium on THP-1 and Gastric Cancer Cell Proliferation
2.2. Effect of DOP on M1 and M2 Subtype Macrophages
2.3. Effect on Transcription Factors in the STAT6/PPAR-r Pathway and its Down-Targeted Proteins in M2-like Subtype Macrophages
2.4. DOP-Inhibited JAGGED1/NOTCH1 Signaling Pathway in M2-like Macrophages
2.5. Effect of CM-DOP on Promoting Apoptosis and Inhibiting Migration in Gastric Cancer Cell
2.6. Effect of DOP on Gastric-Xenografted Tumor Model In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of DOP
4.3. Cell Culture
4.4. THP-1 Cell Differentiation and Treatment
4.5. The Preparation of Conditioned Medium
4.6. Cell Viability Assay
4.7. Measurement of TNF-α and IL-6 via ELISA
4.8. Phenotype Analysis via Flow Cytometry
4.9. Real-Time PCR
4.10. Effect of CM-DOP on Gastric Cancer Cell
4.11. In Vivo Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Ju, W.; Zheng, R.; Zhang, S.; Zeng, H.; Sun, K.; Wang, S.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer statistics in Chinese older people, 2022: Current burden, time trends, and comparisons with the US, Japan, and the Republic of Korea. Sci. China Life Sci. 2022, 65, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. Engl. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tu, S.; Chavda, V.P.; Chen, Z.-S.; Chen, X. Successes and failures of immunotherapy for gastric cancer. Drug Discov. Today 2022, 27, 103343. [Google Scholar] [CrossRef] [PubMed]
- Dedecker, H.; Teuwen, L.-A.; Vandamme, T.; Domen, A.; Prenen, H. The role of Immunotherapy in esophageal and gastric cancer. Clin. Color. Cancer 2023, 22, 175–182. [Google Scholar] [CrossRef]
- Noori, M.; Mahjoubfar, A.; Azizi, S.; Fayyaz, F.; Rezaei, N. Immune checkpoint inhibitors plus chemotherapy versus chemotherapy alone as first-line therapy for advanced gastric and esophageal cancers: A systematic review and meta-analysis. Int. Immunopharmacol. 2022, 113, 109317. [Google Scholar] [CrossRef]
- Fendl, B.; Berghoff, A.S.; Preusser, M.; Maier, B. Macrophage and monocyte subsets as new therapeutic targets in cancer immunotherapy. ESMO Open 2023, 8, 100776. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013, 34, 216–223. [Google Scholar] [CrossRef]
- Mohd Yasin, Z.N.; Mohd Idrus, F.N.; Hoe, C.H.; Yvonne-Tee, G.B. Macrophage polarization in THP-1 cell line and primary monocytes: A systematic review. Differentiation 2022, 128, 67–82. [Google Scholar] [CrossRef]
- Eun, H.S.; Jeong, W.I. Dual Notch signaling in proinflammatory macrophage activation. Hepatology 2016, 63, 1381–1383. [Google Scholar] [CrossRef] [PubMed]
- Mushref, M.; Shah, V.H. Getting a NOTCH-up on Macrophage Activation in Alcoholic Liver Disease. Gastroenterology 2015, 149, 1979–1980. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhang, M.; Xuan, Q.; Wang, Z.; Lian, X.; Zhang, Q. Jagged1 promotes aromatase inhibitor resistance by modulating tumor-associated macrophage differentiation in breast cancer patients. Breast Cancer Res. Treat. 2017, 166, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Sangphech, N.; Keawvilai, P.; Palaga, T. Notch signaling increases PPARγ protein stability and enhances lipid uptake through AKT in IL-4-stimulated THP-1 and primary human macrophages. FEBS Open Bio. 2020, 10, 1082–1095. [Google Scholar] [CrossRef]
- Zhao, J.L.; Huang, F.; He, F.; Gao, C.C.; Liang, S.Q.; Ma, P.F.; Dong, G.Y.; Han, H.; Qin, H.Y. Forced Activation of Notch in Macrophages Represses Tumor Growth by Upregulating miR-125a and Disabling Tumor-Associated Macrophages. Cancer Res. 2016, 76, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.P.; Chen, Y.Y.; An, P.; Rahman, K.; Luan, X.; Zhang, H. Natural products targeting macrophages in tumor microenvironment are a source of potential antitumor agents. Phytomedicine 2023, 109, 154612. [Google Scholar] [CrossRef]
- Eswar, K.; Mukherjee, S.; Ganesan, P.; Rengan, A.K. Immunomodulatory natural polysaccharides: An overview of the mechanisms involved. Eur. Polym. J. 2023, 188, 111935. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.F.; Shen, Z.C.; Zou, Q.; Lin, X.F.; Wang, X.Y. Recent developments on natural polysaccharides as potential anti-gastric cancer substance: Structural feature and bioactivity. Int. J. Biol. Macromol. 2023, 232, 123390. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, J.; Mi, Y.; Tong, L.T.; Lu, C.; Liu, J.M.; Jia, N.; Liu, J.N.; Wang, H.; Fan, B.; et al. Application progress of active ingredients in plants of Dendrobii Caulis and biological models of in protection of gastrointestinal diseases. Chin. Tradit. Herb. Drugs 2021, 52, 7025–7031. [Google Scholar]
- Chu, W.H.; Wang, P.; Ma, Z.; Peng, L.; Wang, Z.M.; Chen, Z.L. Ultrasonic treatment of Dendrobium officinale polysaccharide enhances antioxidant and anti-inflammatory activity in a mouse D-galactose-induced aging model. Food Sci. Nutr. 2022, 10, 2620–2630. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Tao, W.Y.; Wang, M.Z.; Liu, W.; Xing, J.R.; Yang, Y. Dendrobium officinale Xianhu 2 polysaccharide helps forming a healthy gut microbiota and improving host immune system: An in vitro and in vivo study. Food Chem. 2023, 401, 134211. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Wang, K.; Cao, X. Structural characterisation and bioactivity of polysaccharides isolated from fermented Dendrobium officinale. J. Sci. Food Agric. 2022, 102, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Li, C.Y.; Hu, C.; Gong, P.S.; Zhao, S.H. Purification and Structural Characterization of Dendrobium officinale Polysaccharides and Its Activities. Chem. Biodivers. 2021, 18, e2001023. [Google Scholar] [CrossRef]
- Chen, W.H.; Wu, J.J.; Li, X.F.; Lu, J.M.; Wu, W.; Sun, Y.Q.; Zhu, B.; Qin, L.P. Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: A review. Int. J. Biol. Macromol. 2021, 184, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, F.; Ren, X. Inhibitory effect of Dendrobium officinale polysaccharide on human gastric cancer cell xenografts in nude mice. Food Sci. Technol. 2017, 38, 78–83. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ge, J.C.; Zhang, F.Y.; Zha, X.Q.; Liu, J.; Li, Q.M.; Luo, J.P. Dendrobium officinale polysaccharide promotes M1 polarization of TAMs to inhibit tumor growth by targeting TLR2. Carbohydr. Polym. 2022, 292, 119683. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Reedijk, M. Notch Signaling and the Breast Cancer Microenvironment. Adv. Exp. Med. Biol. 2021, 1287, 183–200. [Google Scholar]
- Ma, R.-Y.; Black, A.; Qian, B.-Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022, 43, 546–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Hao, H. Macrophage phenotype-switching in cancer. Eur. J. Pharmacol. 2022, 931, 175229. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Ma, P.; Huang, H.; Tang, Q.; Miao, H.; Fang, Y.; Jiang, N.; Li, Y.; Zhu, Q.; et al. Landscape and perspectives of macrophage -targeted cancer therapy in clinical trials. Mol. Ther. Oncolytics 2022, 24, 799–813. [Google Scholar] [CrossRef]
- Zhu, P.F.; Wang, M.X.; Chen, Z.L.; Yang, L. Targeting the Tumor Microenvironment: A Literature Review of the Novel Anti-Tumor Mechanism of Statins. Front. Oncol. 2021, 11, 761107. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, M.; Wang, C.; Nie, S.; Cui, S.W.; Guo, Q. Acetyl-glucomannan from Dendrobium officinale: Structural modification and immunomodulatory activities. Front. Nutr. 2022, 9, 1016961. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, H.; Chen, J.; He, L.; Du, X.; Zhou, L.; Xiong, Q.; Lai, X.; Yang, Y.; Huang, S.; et al. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2019, 148, 104417. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Y.; Lv, Y.; Li, X.; Chen, L.; Huang, Z.; Zhou, J.; Wang, Y.; Wang, X.; Wang, X.; et al. Dendrobium officinale polysaccharides attenuate uropathogenic Escherichia coli (UPEC)-induced pyroptosis in macrophage cells. Biomed. Pharmacother. 2022, 151, 113098. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Musick, M.; Yu, X. Manipulation of the tumor immuno-microenvironment via TAM-targeted expression of transcription factors. Immunol. Res. 2022, 70, 432–440. [Google Scholar] [CrossRef]
- Binnemars-Postma, K.; Bansal, R.; Storm, G.; Prakash, J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018, 32, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Szanto, A.; Balint, B.L.; Nagy, Z.S.; Barta, E.; Dezso, B.; Pap, A.; Szeles, L.; Poliska, S.; Oros, M.; Evans, R.M.; et al. STAT6 Transcription Factor Is a Facilitator of the Nuclear Receptor PPARγ-Regulated Gene Expression in Macrophages and Dendritic Cells. Immunity 2010, 33, 699–712. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, K.; Kim, M.; Ahn, Y.H.; Kang, J.L. Inhibition of STAT6 Activation by AS1517499 Inhibits Expression and Activity of PPARγ in Macrophages to Resolve Acute Inflammation in Mice. Biomolecules 2022, 12, 447. [Google Scholar] [CrossRef]
- Yao, Z.M.; Zhang, X.; Yang, S.X.; Zhu, J.J.; Hu, X.X.; Shen, T. The role of STAT-6/KLF-4/PPAR-γ activation in alveolar macrophage polarization changes in silica-induced pulmonary fibrosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2022, 40, 481–486. [Google Scholar]
- Jun, I.; Kim, B.-R.; Park, S.Y.; Lee, H.; Kim, J.; Kim, E.K.; Seo, K.Y.; Kim, T.-i. Interleukin-4 stimulates lipogenesis in meibocytes by activating the STAT6/PPARγ signaling pathway. Ocul. Surf. 2020, 18, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Jackstadt, R.; Hooff, S.; Leach, J.D.; Cortes-Lavaud, X.; Sansom, O.J. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell 2019, 36, 319–336.e7. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Li, X.; Feng, G.; Bai, Y. The Impact of NOTCH Pathway Alteration on Tumor Microenvironment and Clinical Survival of Immune Checkpoint Inhibitors in NSCLC. Front. Immunol. 2021, 12, 638763. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; He, F.; Feng, F.; Liu, X.W.; Dong, G.Y.; Qin, H.Y.; Hu, X.B.; Zheng, M.H.; Liang, L.; Feng, L.; et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010, 70, 4840–4849. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, J.L.; Zheng, Q.J.; Jiang, X.; Tian, J.; Liang, S.Q.; Guo, H.W.; Qin, H.Y.; Liang, Y.M.; Han, H. Notch Signaling Modulates Macrophage Polarization and Phagocytosis through Direct Suppression of Signal Regulatory Protein α Expression. Front. Immunol. 2018, 9, 1744. [Google Scholar] [CrossRef]
- Hans, C.P.; Sharma, N.; Sen, S.; Zeng, S.; Dev, R.; Jiang, Y.; Mahajan, A.; Joshi, T. Transcriptomics Analysis Reveals New Insights into the Roles of Notch1 Signaling on Macrophage Polarization. Sci. Rep. 2019, 9, 7999. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, M.; Tuorkey, M.; Sak, K.; Parashar, N.C.; Barwal, T.S.; Sharma, U.; Iqubal, A.; Parashar, G.; et al. NOTCH signaling: Journey of an evolutionarily conserved pathway in driving tumor progression and its modulation as a therapeutic target. Crit. Rev. Oncol. Hematol. 2021, 164, 103403. [Google Scholar] [CrossRef]
- Wang, T.; Lu, H.H. Ganoderic acid A inhibits ox-LDL-induced THP-1-derived macrophage inflammation and lipid deposition via Notch1/PPAR gamma/CD36 signaling. Adv. Clin. Exp. Med. 2021, 30, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Inagaki, K.; Kunisho, S.; Takigawa, H.; Yuge, R.; Oka, S.; Tanaka, S.; Shimamoto, F.; Chayama, K.; Kitadai, Y. Role of tumor-associated macrophages at the invasive front in human colorectal cancer progression. Cancer Sci. 2021, 112, 2692–2704. [Google Scholar] [CrossRef]
- Liang, M.; Zhao, Z.L.; Yu, G.T.; Wu, L.; Sun, Z.J. γ-Secretase inhibitor reduces immunosuppressive cells and enhances tumour immunity in head and neck squamous cell carcinoma. Int. J. Cancer 2018, 142, 999–1009. [Google Scholar]
- Yin, J.-Z.; Shi, X.-Q.; Wang, M.-D.; Du, H.; Zhao, X.-W.; Li, B.; Yang, M.-H. Arsenic trioxide elicits anti-tumor activity by inhibiting polarization of M2-like tumor-associated macrophages via Notch signaling pathway in lung adenocarcinoma. Int. Immunopharmacol. 2023, 117, 109899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Y.Z.; Wang, G.Y.; Ge, S.C.; Liu, H.N. Dendrobium Officinale Polysaccharides Protect against MNNG-Induced PLGC in Rats via Activating the NRF2 and Antioxidant Enzymes HO-1 and NQO-1. Oxidative Med. Cell Longev. 2019, 2019, 9310245. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| IL-6 | TACATCCTCGACGGCATCTC | CCATCTTTGGAAGGTTCAGG |
| Fizz1 | GCAAGAAGCTCTCGTGTGCTAGTG | CGAACCACAGCCATAGCCACAAG |
| ARG 1 | CACACCAGCTACTGGCACACC | GCAACTGCTGTGTTCACTGTTCG |
| TGM2 | GCAGTGACTTTGACGTCTTTGCCC | GTAGCTGTTGATAACTGGCTCCACG |
| CXCL9 | GCTGGTTCTGATTGGAGTGC | GAAGGGCTTGGGGCAAATTG |
| CXCL10 | CCTTATCTTTCTGACTCTAAGTGG | CTAAAGACCTTGGATTAACAGG |
| IRF1 | GGAAGGGAAATTACCTGAGG | CTCCAGGTTCATTGAGTAGG |
| ALOX15 | CAGATGTCCATCACTTGGCAG | CTCCTCCCTGAACTTCTTCAG |
| STAT1 | CAATGCTTGCTTGGATCAGC | GTGATAGGGTCATGTTGCTAGG |
| IL1β | AATGATGGCTTATTACAGTGGCA | GTCGGAGATTCGTAGCTGGA |
| PPAR-r | TTCAGAAATGCCTTGCAGTG | CCAACAGCTTCTCCTTCTCG |
| TNF-a | GGAGAAGGGTGACCGACTC | TGGGAAGGTTGGATGTTCGT |
| NOTCH1 | GAGGCGTGGCAGACTATGC | CTTGTACTCCGTCAGCGTGA |
| JAGGDE1 | TGACCAGAATGGCAACAAAA | GTTGGGTCCTGAATACCCCT |
| β-actin | GTGGCCGAGGACTTTGATTG | CCTGTAACAACGCATCTCATATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Lu, X.; Huang, H.; Yao, Y.; Liu, H.; Sun, Y. Dendrobium officinale polysaccharide Converts M2 into M1 Subtype Macrophage Polarization via the STAT6/PPAR-r and JAGGED1/NOTCH1 Signaling Pathways to Inhibit Gastric Cancer. Molecules 2023, 28, 7062. https://doi.org/10.3390/molecules28207062
Zhao Y, Lu X, Huang H, Yao Y, Liu H, Sun Y. Dendrobium officinale polysaccharide Converts M2 into M1 Subtype Macrophage Polarization via the STAT6/PPAR-r and JAGGED1/NOTCH1 Signaling Pathways to Inhibit Gastric Cancer. Molecules. 2023; 28(20):7062. https://doi.org/10.3390/molecules28207062
Chicago/Turabian StyleZhao, Yi, Xuefeng Lu, Hongxia Huang, Yao Yao, Hongning Liu, and Youzhi Sun. 2023. "Dendrobium officinale polysaccharide Converts M2 into M1 Subtype Macrophage Polarization via the STAT6/PPAR-r and JAGGED1/NOTCH1 Signaling Pathways to Inhibit Gastric Cancer" Molecules 28, no. 20: 7062. https://doi.org/10.3390/molecules28207062
APA StyleZhao, Y., Lu, X., Huang, H., Yao, Y., Liu, H., & Sun, Y. (2023). Dendrobium officinale polysaccharide Converts M2 into M1 Subtype Macrophage Polarization via the STAT6/PPAR-r and JAGGED1/NOTCH1 Signaling Pathways to Inhibit Gastric Cancer. Molecules, 28(20), 7062. https://doi.org/10.3390/molecules28207062