A Review of Chondroitin Sulfate’s Preparation, Properties, Functions, and Applications
Abstract
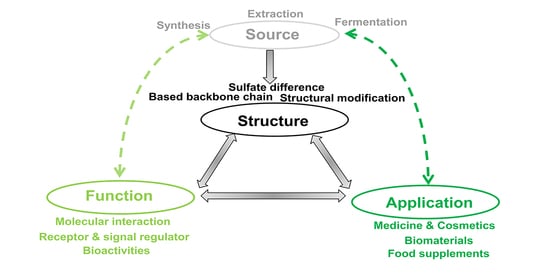
:1. Introduction
2. Preparation of CS
2.1. The Sources of CS

2.2. CS Extraction from Cartilage
2.3. Enzymatic and Chemical Synthesis and Fermentation for CS

2.4. Summary of CS Preparation Methods
3. Properties of Chondroitin Sulfate
3.1. Structural Properties
3.2. The Complex Properties of CS

3.3. Summary of the Properties of CS
4. Applications of Chondroitin Sulfate
4.1. Functions of Chondroitin Sulfate
4.2. Applications of Chondroitin Sulfate
4.3. Summary of CS Applications
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mikami, T.; Kitagawa, H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta 2013, 1830, 4719–4733. [Google Scholar] [CrossRef] [PubMed]
- Poh, Z.W.; Gan, C.H.; Lee, E.J.; Guo, S.; Yip, G.W.; Lam, Y. Divergent Synthesis of Chondroitin Sulfate Disaccharides and Identification of Sulfate Motifs that Inhibit Triple Negative Breast Cancer. Sci. Rep. 2015, 5, 14355. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, M.; Jain, A.; Hurkat, P.; Jain, S.K. Chondroitin sulphate: A focus on osteoarthritis. Glycoconj. J. 2016, 33, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, F.; Pang, X.; Tang, B.; Lin, L. Synthesis of chondroitin sulfate magnesium for osteoarthritis treatment. Carbohydr. Polym. 2019, 212, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.B.; Liu, N.; Hu, N.; Wen, C.Y.; Tang, B. Synthesis of strontium chondroitin sulfate and the evaluation of its capability to attenuate osteoarthritis. Carbohydr. Polym. 2017, 170, 217–225. [Google Scholar] [CrossRef]
- Fenbo, M.; Xingyu, X.; Bin, T. Strontium chondroitin sulfate/silk fibroin blend membrane containing microporous structure modulates macrophage responses for guided bone regeneration. Carbohydr. Polym. 2019, 213, 266–275. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, C.; Mo, H.; Zhang, H.; Qin, X.; Li, J.; Zhang, Z.; Richel, A. Fabrication of chondroitin sulfate calcium complex and its chondrocyte proliferation in vitro. Carbohydr. Polym. 2021, 254, 117282. [Google Scholar] [CrossRef]
- Li, C.; Sahu, S.; Kou, G.; Jagadeesan, N.; Joseph, T.P.; Li Lin, S.; Schachner, M. Chondroitin 6-sulfate-binding peptides improve recovery in spinal cord-injured mice. Eur. J. Pharmacol. 2021, 910, 174421. [Google Scholar] [CrossRef]
- Volpi, N. Chondroitin Sulfate Safety and Quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef]
- Shi, Y.; Meng, Y.-C.; Li, J.R.; Chen, J.; Liu, Y.H.; Bai, X. Chondroitin Sulfate: Extraction, Purification, Microbial and Chemical Synthesis. J. Chem. Technol. Biotechnol. 2014, 89, 1445–1465. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, W.; Wang, Y.; Sheng, J.; Kang, Z.J.G.C. Biosynthesis of non-animal chondroitin sulfate from methanol using genetically engineered Pichia pastoris. Green. Chem. 2021, 23, 4365–4374. [Google Scholar] [CrossRef]
- Kubový, P.; Mensíková, L.; Kůrková, E.; Lopot, F.; Hojka, V.; Jelen, K. Influence of SYSADOA group chemicals on progression of human knee joint osteoarthritis: New objective evaluation method-measuring of rheological properties in vivo. Neuroendocrinol. Lett. 2012, 33, 651–659. [Google Scholar] [PubMed]
- Schneider, H.; Maheu, E.; Cucherat, M. Symptom-modifying effect of chondroitin sulfate in knee osteoarthritis: A meta-analysis of randomized placebo-controlled trials performed with structum(®). Open Rheumatol. J. 2012, 6, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, M.; Sánchez-García, A.; Vilchez-Cavazos, F.; Acosta-Olivo, C.A.; Peña-Martínez, V.M.; Simental-Mendía, L.E. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis: A systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol. Int. 2018, 38, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Liu, J.; Zhou, N. Efficacy and safety of the combination of glucosamine and chondroitin for knee osteoarthritis: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2023, 143, 409–421. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef]
- Stellavato, A.; Restaino, O.F.; Vassallo, V.; Finamore, R.; Ruosi, C.; Cassese, E.; De Rosa, M.; Schiraldi, C. Comparative Analyses of Pharmaceuticals or Food Supplements Containing Chondroitin Sulfate: Are Their Bioactivities Equivalent? Adv. Ther. 2019, 36, 3221–3237. [Google Scholar] [CrossRef]
- Li, W.; Kobayashi, T.; Moroi, S.; Kotake, H.; Ikoma, T.; Saeki, H.; Ura, K.; Takagi, Y. Anti-obesity effects of chondroitin sulfate oligosaccharides from the skate Raja pulchra. Carbohydr. Polym. 2019, 214, 303–310. [Google Scholar] [CrossRef]
- Shang, Q.; Shi, J.; Song, G.; Zhang, M.; Cai, C.; Hao, J.; Li, G.; Yu, G. Structural modulation of gut microbiota by chondroitin sulfate and its oligosaccharide. Int. J. Biol. Macromol. 2016, 89, 489–498. [Google Scholar] [CrossRef]
- Luo, X.M.; Fosmire, G.J.; Leach, R.M., Jr. Chicken keel cartilage as a source of chondroitin sulfate. Poult. Sci. 2002, 81, 1086–1089. [Google Scholar] [CrossRef]
- Nakano, T.; Srichamroen, A.; Ozimek, L. Extraction of aggrecan-peptide from cartilage by tissue autolysis. Recent. Pat. Food Nutr. Agric. 2014, 6, 54–59. [Google Scholar] [CrossRef]
- Shin, S.C.; You, S.J.; An, B.K.; Kang, C.W. Study on Extraction of Mucopolysaccharide-protein Containing Chondroitin Sulfate from Chicken Keel Cartilage Electrophoresis. Asian-Australas. J. Anim. Sci. 2006, 19, 601–604. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, C.; Jia, W.; Qin, X.; Xu, X.; Ye, M.; Mo, H.; Richel, A. Liquefaction of chicken sternal cartilage by steam explosion to isolate chondroitin sulfate. Carbohydr. Polym. 2019, 215, 73–81. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, C.; Jia, W.; Qin, X.; Cui, Z.; Mo, H.; Richel, A. Co-production of chondroitin sulfate and peptide from liquefied chicken sternal cartilage by hot-pressure. Carbohydr. Polym. 2019, 222, 115015. [Google Scholar] [CrossRef]
- Srichamroen, A.; Nakano, T.; Pietrasik, Z.; Ozimek, L.; Betti, M. Chondroitin sulfate extraction from broiler chicken cartilage by tissue autolysis. LWT-Food Sci. Technol. 2013, 50, 607–612. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Q.; Zhang, C.; Jia, W.; Han, L.; Yu, Q. Chicken leg bone as a source of chondroitin sulfate. Carbohydr. Polym. 2019, 207, 191–199. [Google Scholar] [CrossRef]
- Nakano, T.; Pietrasik, Z.; Ozimek, L.; Betti, M.J.P.B. Extraction, isolation and analysis of chondroitin sulfate from broiler chicken biomass. Process Biochem. 2012, 47, 1909–1918. [Google Scholar] [CrossRef]
- Nakano, T.; Ozimek, L. Chondroitin sulphate distribution in broiler chicken carcasses. Br. Poult. Sci. 2014, 55, 54–58. [Google Scholar] [CrossRef]
- Zou, Z.; Wei, M.; Fang, J.; Dai, W.; Sun, T.; Liu, Q.; Gong, G.; Liu, Y.; Song, S.; Ma, F.; et al. Preparation of chondroitin sulfates with different molecular weights from bovine nasal cartilage and their antioxidant activities. Int. J. Biol. Macromol. 2020, 152, 1047–1055. [Google Scholar] [CrossRef]
- Muthusamy, A.; Achur, R.N.; Valiyaveettil, M.; Madhunapantula, S.V.; Kakizaki, I.; Bhavanandan, V.P.; Gowda, C.D. Structural characterization of the bovine tracheal chondroitin sulfate chains and binding of Plasmodium falciparum-infected erythrocytes. Glycobiology 2004, 14, 635–645. [Google Scholar] [CrossRef]
- Volpi, N. Purification of heparin, dermatan sulfate and chondroitin sulfate from mixtures by sequential precipitation with various organic solvents. J. Chromatogr. B Biomed. Appl. 1996, 685, 27–34. [Google Scholar] [CrossRef]
- Achur, R.N.; Muthusamy, A.; Madhunapantula, S.V.; Bhavanandan, V.P.; Seudieu, C.; Channe Gowda, D. Chondroitin sulfate proteoglycans of bovine cornea: Structural characterization and assessment for the adherence of Plasmodium falciparum-infected erythrocytes. Biochim. Biophys. Acta 2004, 1701, 109–119. [Google Scholar] [CrossRef]
- Rees, S.G.; Flannery, C.R.; Little, C.B.; Hughes, C.E.; Caterson, B.; Dent, C.M. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem. J. 2000, 350 Pt 1, 181–188. [Google Scholar] [CrossRef]
- Hitchcock, A.M.; Yates, K.E.; Costello, C.E.; Zaia, J. Comparative glycomics of connective tissue glycosaminoglycans. Proteomics 2008, 8, 1384–1397. [Google Scholar] [CrossRef]
- da Cunha, A.L.; Aguiar, J.A.K.; Correa da Silva, F.S.; Michelacci, Y.M. Do chondroitin sulfates with different structures have different activities on chondrocytes and macrophages? Int. J. Biol. Macromol. 2017, 103, 1019–1031. [Google Scholar] [CrossRef]
- Sampaio Lde, O.; Bayliss, M.T.; Hardingham, T.E.; Muir, H. Dermatan sulphate proteoglycan from human articular cartilage. Variation in its content with age and its structural comparison with a small chondroitin sulphate proteoglycan from pig laryngeal cartilage. Biochem. J. 1988, 254, 757–764. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, G.; Gao, H. Isolation and purification of chondroitin sulfate from porcine cartilage. Chin. J. Biochem. Pharm. 2004, 25, 144–146. [Google Scholar]
- Yanagishita, M.; Rodbard, D.; Hascall, V.C. Isolation and characterization of proteoglycans from porcine ovarian follicular fluid. J. Biol. Chem. 1979, 254, 911–920. [Google Scholar] [CrossRef]
- Watanabe, M.; Nojima, M.; Shibata, T.; Hamada, M. Maturation-related biochemical changes in swine anterior cruciate ligament and tibialis posterior tendon. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1994, 12, 672–682. [Google Scholar] [CrossRef]
- Nakano, T.; Sunwoo, H.H.; Li, X.; Price, M.A.; Sim, J.S.; Chemistry, F. Study of Sulfated Glycosaminoglycans from Porcine Skeletal Muscle Epimysium Including Analysis of Iduronosyl and Glucuronosyl Residues in Galactosaminoglycan Fractions. J. Agric. Food Chem. 1996, 44, 1424–1434. [Google Scholar] [CrossRef]
- Seno, N.; Anno, K.; Yaegashi, Y.; Okuyama, T. Microheterogeneity of chondroitin sulfates from various cartilages. Connect. Tissue Res. 1975, 3, 87–96. [Google Scholar] [CrossRef]
- Sunwoo, H.H.; Nakano, T.; Hudson, R.J.; Sim, J.S. Isolation, characterization and localization of glycosaminoglycans in growing antlers of wapiti (Cervus elaphus). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1998, 120, 273–283. [Google Scholar] [CrossRef]
- Scott, J.E.; Hughes, E.W. Chondroitin sulphate from fossilized antlers. Nature 1981, 291, 580–581. [Google Scholar] [CrossRef]
- Zhao, Q.C.; Kiyohara, H.; Nagai, T.; Yamada, H. Structure of the complement-activating proteoglycan from the pilose antler of Cervus nippon Temminck. Carbohydr. Res. 1992, 230, 361–372. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González Mdel, P.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mourão, P.A. Occurrence of a unique fucose-branched chondroitin sulfate in the body wall of a sea cucumber. J. Biol. Chem. 1988, 263, 18176–18183. [Google Scholar] [CrossRef]
- Kinoshita-Toyoda, A.; Yamada, S.; Haslam, S.M.; Khoo, K.H.; Sugiura, M.; Morris, H.R.; Dell, A.; Sugahara, K. Structural determination of five novel tetrasaccharides containing 3-O-sulfated D-glucuronic acid and two rare oligosaccharides containing a beta-D-glucose branch isolated from squid cartilage chondroitin sulfate E. Biochemistry 2004, 43, 11063–11074. [Google Scholar] [CrossRef]
- Sim, J.S.; Im, A.R.; Cho, S.M.; Jang, H.J.; Jin, H.J.; Kim, Y.S. Evaluation of chondroitin sulfate in shark cartilage powder as a dietary supplement: Raw materials and finished products. Food Chem. 2007, 101, 532–539. [Google Scholar] [CrossRef]
- Ping, W.; Tang, J. Solvent-free mechanochemical extraction of chondroitin sulfate from shark cartilage. Chem. Eng. Process. Process Intensif. 2009, 48, 1187–1191. [Google Scholar]
- Sugahara, K.; Nadanaka, S.; Takeda, K.; Kojima, T. Structural analysis of unsaturated hexasaccharides isolated from shark cartilage chondroitin sulfate D that are substrates for the exolytic action of chondroitin ABC lyase. Eur. J. Biochem. 1996, 239, 871–880. [Google Scholar] [CrossRef]
- Lignot, B.; Lahogue, V.; Bourseau, P. Enzymatic extraction of chondroitin sulfate from skate cartilage and concentration-desalting by ultrafiltration. J. Biotechnol. 2003, 103, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Mi, S.; Li, J.; Gao, J.; Wang, X.; Sang, Y. Purification, characterisation and antioxidant activities of chondroitin sulphate extracted from Raja porosa cartilage. Carbohydr. Polym. 2020, 241, 116306. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.K.; Kobayashi, T.; Mizumoto, S.; Narumi, M.; Kudo, Y.; Yamada, S.; Sugahara, K. Isolation and characterization of a novel chondroitin sulfate from squid liver integument rich in N-acetylgalactosamine(4,6-disulfate) and glucuronate(3-sulfate) residues. Carbohydr. Res. 2009, 344, 1526–1532. [Google Scholar] [CrossRef]
- Chen, X.E.; Fang, X.B.; Hui, Y.; Ru, S.J.F.S. Technology. Study on the extraction technology of chondroitin sulfate from squid cartilage. Food Sci. Technol. 2008, 33, 214–217. [Google Scholar]
- Kinoshita, A.; Yamada, S.; Haslam, S.M.; Morris, H.R.; Dell, A.; Sugahara, K. Novel tetrasaccharides isolated from squid cartilage chondroitin sulfate E contain unusual sulfated disaccharide units GlcA(3-O-sulfate)beta1-3GalNAc(6-O-sulfate) or GlcA(3-O-sulfate)beta1-3GalNAc. J. Biol. Chem. 1997, 272, 19656–19665. [Google Scholar] [CrossRef]
- Gui, M.; Song, J.; Zhang, L.; Wang, S.; Wu, R.; Ma, C.; Li, P. Chemical characteristics and antithrombotic effect of chondroitin sulfates from sturgeon skull and sturgeon backbone. Carbohydr. Polym. 2015, 123, 454–460. [Google Scholar] [CrossRef]
- Zhao, T.; Zhou, Y.; Mao, G.; Zou, Y.; Zhao, J.; Bai, S.; Yang, L.; Wu, X. Extraction, purification and characterisation of chondroitin sulfate in Chinese sturgeon cartilage. J. Sci. Food Agric. 2013, 93, 1633–1640. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Sun, Y.; Chen, L.; Wang, J.; Liang, L. Chondroitin sulfate from sturgeon bone protects rat chondrocytes from hydrogen peroxide-induced damage by maintaining cellular homeostasis through enhanced autophagy. Int. J. Biol. Macromol. 2020, 164, 2761–2768. [Google Scholar] [CrossRef]
- Lee, K.B.; Kim, J.S.; Kwak, S.T.; Sim, W.; Kwak, J.H.; Kim, Y.S. Isolation and identification of chondroitin sulfates from the mud snail. Arch. Pharmacal Res. 1998, 21, 555–558. [Google Scholar] [CrossRef]
- Field, I.C.; Meekan, M.G.; Buckworth, R.C.; Bradshaw, C.J. Chapter 4. Susceptibility of sharks, rays and chimaeras to global extinction. Adv. Mar. Biol. 2009, 56, 275–363. [Google Scholar] [CrossRef]
- García, V.B.; Lucifora, L.O.; Myers, R.A. The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras. Proceedings. Biol. Sci. 2008, 275, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Maccari, F.; Galeotti, F.; Volpi, N. Isolation and structural characterization of chondroitin sulfate from bony fishes. Carbohydr. Polym. 2015, 129, 143–147. [Google Scholar] [CrossRef]
- Gargiulo, V.; Lanzetta, R.; Parrilli, M.; De Castro, C. Structural analysis of chondroitin sulfate from Scyliorhinus canicula: A useful source of this polysaccharide. Glycobiology 2009, 19, 1485–1491. [Google Scholar] [CrossRef]
- Kim, S.B.; Ji, C.I.; Woo, J.W.; Do, J.R.; Cho, S.-M.; Lee, Y.-B.; Kang, S.-N.; Park, J.-H. Simplified purification of chondroitin sulphate from scapular cartilage of shortfin mako shark (Isurus oxyrinchus). Int. J. Food Sci. Technol. 2015, 47, 91–99. [Google Scholar] [CrossRef]
- Akram, A.N.; Zhang, C. Effect of ultrasonication on the yield, functional and physicochemical characteristics of collagen-II from chicken sternal cartilage. Food Chem. 2020, 307, 125544. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zheng, L.; Su, G.; Luo, D.; Lai, C.; Zhao, M. Protein solubility, secondary structure and microstructure changes in two types of undenatured type II collagen under different gastrointestinal digestion conditions. Food Chem. 2021, 343, 128555. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, S.; Qiao, M.; Jiao, R.; Li, J.; Song, P.; Zhang, X.; Huang, H. Synthesis of structurally defined chondroitin sulfate: Paving the way to the structure-activity relationship studies. Carbohydr. Polym. 2020, 248, 116796. [Google Scholar] [CrossRef]
- Li, J.; Su, G.; Liu, J. Enzymatic Synthesis of Homogeneous Chondroitin Sulfate Oligosaccharides. Angew. Chem. (Int. Ed. Engl.) 2017, 56, 11784–11787. [Google Scholar] [CrossRef]
- Sugiura, N.; Shimokata, S.; Minamisawa, T.; Hirabayashi, J.; Kimata, K.; Watanabe, H. Sequential synthesis of chondroitin oligosaccharides by immobilized chondroitin polymerase mutants. Glycoconj. J. 2008, 25, 521–530. [Google Scholar] [CrossRef]
- Fujikawa, S.; Ohmae, M.; Kobayashi, S. Enzymatic synthesis of chondroitin 4-sulfate with well-defined structure. Biomacromolecules 2005, 6, 2935–2942. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fujikawa, S.; Ohmae, M. Enzymatic synthesis of chondroitin and its derivatives catalyzed by hyaluronidase. J. Am. Chem. Soc. 2003, 125, 14357–14369. [Google Scholar] [CrossRef]
- Frost, G.I.; Tony, C.; Robert, S.J.T. The Hyaluronidases: A Chemical, Biological and Clinical Overview. Trends Glycosci. Glycotechnol. 1996, 8, 419–434. [Google Scholar] [CrossRef]
- Marković-Housley, Z.; Miglierini, G.; Soldatova, L.; Rizkallah, P.J.; Müller, U.; Schirmer, T. Crystal structure of hyaluronidase, a major allergen of bee venom. Structure 2000, 8, 1025–1035. [Google Scholar] [CrossRef]
- Mende, M.; Bednarek, C.; Wawryszyn, M.; Sauter, P.; Biskup, M.B.; Schepers, U.; Bräse, S. Chemical Synthesis of Glycosaminoglycans. Chem. Rev. 2016, 116, 8193–8255. [Google Scholar] [CrossRef]
- He, H.; Chen, D.; Li, X.; Li, C.; Zhao, J.H.; Qin, H.B. Synthesis of trisaccharide repeating unit of fucosylated chondroitin sulfate. Org. Biomol. Chem. 2019, 17, 2877–2882. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Lin, L.; Yao, W.; Zhao, J.; Wu, M.; Li, Z. Synthesis of Fucosylated Chondroitin Sulfate Nonasaccharide as a Novel Anticoagulant Targeting Intrinsic Factor Xase Complex. Angew. Chem. (Int. Ed. Engl.) 2018, 57, 12880–12885. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, P.; Wang, L.; Sun, T.; Cai, C.; Yu, G. Synthesis and Properties of Functional Glycomimetics through Click Grafting of Fucose onto Chondroitin Sulfates. Biomacromolecules 2019, 20, 3798–3808. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Q.; Zhang, G.; Zhang, X.; Zhao, Z.; Lei, P. An Approach to Synthesize Chondroitin Sulfate-E (CS-E) Oligosaccharide Precursors. J. Org. Chem. 2018, 83, 5897–5908. [Google Scholar] [CrossRef]
- Matsushita, K.; Nakata, T.; Tamura, J. The application of 2,2,2-trichloroethyl sulfate to the synthesis of chondroitin sulfate C and D. Carbohydr. Res. 2015, 406, 76–85. [Google Scholar] [CrossRef]
- Yao, W.; Zhu, Y.; Zhang, X.; Sha, M.; Meng, X.; Li, Z. Semisynthesis of Chondroitin Sulfate E Tetrasaccharide from Hyaluronic Acid. J. Org. Chem. 2018, 83, 14069–14077. [Google Scholar] [CrossRef]
- Chng, Y.S.; Tristan, G.; Yip, G.W.; Lam, Y. Protecting-Group-Free Synthesis of Chondroitin 6-Sulfate Disaccharide and Tetrasaccharide. Org. Lett. 2019, 21, 4559–4562. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Xu, X.; Tan, Y.; Liu, X.W.; Fang, J. Chemoenzymatic synthesis of homogeneous chondroitin polymers and its derivatives. Carbohydr. Polym. 2020, 232, 115822. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Q.; Huang, H.; Wang, H.; Wang, Y.; Du, G.; Chen, J.; Kang, Z. A microbial-enzymatic strategy for producing chondroitin sulfate glycosaminoglycans. Biotechnol. Bioeng. 2018, 115, 1561–1570. [Google Scholar] [CrossRef]
- Rodriguez, M.L.; Jann, B.; Jann, K. Structure and serological characteristics of the capsular K4 antigen of Escherichia coli O5:K4:H4, a fructose-containing polysaccharide with a chondroitin backbone. Eur. J. Biochem. 1988, 177, 117–124. [Google Scholar] [CrossRef]
- Manzoni, M.; Bergomi, S.; Molinari, F.; Cavazzoni, V.J.B.L. Production and purification of an extracellularly produced K4 polysaccharide from Escherichia coli. Biotechnol. Lett. 1996, 18, 383–386. [Google Scholar] [CrossRef]
- Schiraldi, C.; Cimini, D.; De Rosa, M. Production of chondroitin sulfate and chondroitin. Appl. Microbiol. Biotechnol. 2010, 87, 1209–1220. [Google Scholar] [CrossRef]
- Restaino, O.F.; Cimini, D.; De Rosa, M.; Catapano, A.; De Rosa, M.; Schiraldi, C. High cell density cultivation of Escherichia coli K4 in a microfiltration bioreactor: A step towards improvement of chondroitin precursor production. Microb. Cell Factories 2011, 10, 10. [Google Scholar] [CrossRef]
- Rimler, R.B.; Register, K.B.; Magyar, T.; Ackermann, M.R. Influence of chondroitinase on indirect hemagglutination titers and phagocytosis of Pasteurella multocida serogroups A, D and F. Vet. Microbiol. 1995, 47, 287–294. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Q.; Liu, J.; Chen, J. A Screening Method of Chondroitin Sulfate Productions Train and Fermentation Chondroitin Sulfate. CN Patent ZL201110127831.1, 18 May 2011. [Google Scholar]
- Jin, P.; Zhang, L.; Yuan, P.; Kang, Z.; Du, G.; Chen, J. Efficient biosynthesis of polysaccharides chondroitin and heparosan by metabolically engineered Bacillus subtilis. Carbohydr. Polym. 2016, 140, 424–432. [Google Scholar] [CrossRef]
- Cimini, D.; Restaino, O.F.; Schiraldi, C. Microbial production and metabolic engineering of chondroitin and chondroitin sulfate. Emerg. Top. Life Sci. 2018, 2, 349–361. [Google Scholar] [CrossRef]
- Rigg, G.P.; Barrett, B.; Roberts, I.S. The localization of KpsC, S and T, and KfiA, C and D proteins involved in the biosynthesis of the Escherichia coli K5 capsular polysaccharide: Evidence for a membrane-bound complex. Microbiol. (Read. Engl.) 1998, 144 Pt 10, 2905–2914. [Google Scholar] [CrossRef]
- Cimini, D.; Russo, R.; D’Ambrosio, S.; Dello Iacono, I.; Rega, C.; Carlino, E.; Argenzio, O.; Russo, L.; D’Abrosca, B.; Chambery, A.; et al. Physiological characterization and quantitative proteomic analyses of metabolically engineered E. coli K4 strains with improved pathways for capsular polysaccharide biosynthesis. Biotechnol. Bioeng. 2018, 115, 1801–1814. [Google Scholar] [CrossRef]
- Ninomiya, T.; Sugiura, N.; Tawada, A.; Sugimoto, K.; Watanabe, H.; Kimata, K. Molecular cloning and characterization of chondroitin polymerase from Escherichia coli strain K4. J. Biol. Chem. 2002, 277, 21567–21575. [Google Scholar] [CrossRef]
- Liu, J.; Yang, A.; Liu, J.; Ding, X.; Liu, L.; Shi, Z. KfoE encodes a fructosyltransferase involved in capsular polysaccharide biosynthesis in Escherichia coli K4. Biotechnol. Lett. 2014, 36, 1469–1477. [Google Scholar] [CrossRef]
- Zhu, H.M.; Sun, B.; Li, Y.J.; Meng, D.H.; Zheng, S.; Wang, T.T.; Wang, F.S.; Sheng, J.Z. KfoA, the UDP-glucose-4-epimerase of Escherichia coli strain O5:K4:H4, shows preference for acetylated substrates. Appl. Microbiol. Biotechnol. 2018, 102, 751–761. [Google Scholar] [CrossRef]
- Petrucci, F.; Zoppetti, G.; Oreste, P.; Cipolletti, G.J.W. Process for the Preparation of the Polysaccharides K4 and K5 from Escherichia coli. PCT Patent WO0102597 (A1), 11 January 2001. [Google Scholar]
- Cimini, D.; Restaino, O.F.; Catapano, A.; De Rosa, M.; Schiraldi, C. Production of capsular polysaccharide from Escherichia coli K4 for biotechnological applications. Appl. Microbiol. Biotechnol. 2010, 85, 1779–1787. [Google Scholar] [CrossRef]
- Schiraldi, C.; Alfano, A.; Cimini, D.; Rosa, M.D.; Panariello, A.; Restaino, O.F.; Rosa, M.D. Application of a 22L scale membrane bioreactor and cross-flow ultrafiltration to obtain purified chondroitin. Biotechnol. Prog. 2012, 28, 1012–1018. [Google Scholar] [CrossRef]
- Restaino, O.F.; di Lauro, I.; Cimini, D.; Carlino, E.; De Rosa, M.; Schiraldi, C. Monosaccharide precursors for boosting chondroitin-like capsular polysaccharide production. Appl. Microbiol. Biotechnol. 2013, 97, 1699–1709. [Google Scholar] [CrossRef]
- Jolly, J.F.; Klimaszewski, K.; Nakanishi, Y.; Matsubara, H.; Takahashi, T.; Nishio, K. Microbial-Derived Chondroitin Sulfate. US Patent 20100063001(A1), 8 August 2015. [Google Scholar]
- Cimini, D.; De Rosa, M.; Viggiani, A.; Restaino, O.F.; Carlino, E.; Schiraldi, C. Improved fructosylated chondroitin production by kfoC overexpression in E. coli K4. J. Biotechnol. 2010, 150, 324–331. [Google Scholar] [CrossRef]
- Zanfardino, A.; Restaino, O.F.; Notomista, E.; Cimini, D.; Schiraldi, C.; De Rosa, M.; De Felice, M.; Varcamonti, M. Isolation of an Escherichia coli K4 kfoC mutant over-producing capsular chondroitin. Microb. Cell Factories 2010, 9, 34. [Google Scholar] [CrossRef]
- Cimini, D.; De Rosa, M.; Carlino, E.; Ruggiero, A.; Schiraldi, C. Homologous overexpression of RfaH in E. coli K4 improves the production of chondroitin-like capsular polysaccharide. Microb. Cell Factories 2013, 12, 46. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, A.; Zou, W.; Duan, Z.; Liu, J.; Chen, J.; Liu, L. Transcriptional engineering of Escherichia coli K4 for fructosylated chondroitin production. Biotechnol. Prog. 2013, 29, 1140–1149. [Google Scholar] [CrossRef]
- Cimini, D.; Fantaccione, S.; Volpe, F.; De Rosa, M.; Restaino, O.F.; Aquino, G.; Schiraldi, C. IS2-mediated overexpression of kfoC in E. coli K4 increases chondroitin-like capsular polysaccharide production. Appl. Microbiol. Biotechnol. 2014, 98, 3955–3964. [Google Scholar] [CrossRef]
- Cimini, D.; Carlino, E.; Giovane, A.; Argenzio, O.; Dello Iacono, I.; De Rosa, M.; Schiraldi, C. Engineering a branch of the UDP-precursor biosynthesis pathway enhances the production of capsular polysaccharide in Escherichia coli O5:K4:H4. Biotechnol. J. 2015, 10, 1307–1315. [Google Scholar] [CrossRef]
- He, W.; Fu, L.; Li, G.; Andrew Jones, J.; Linhardt, R.J.; Koffas, M. Production of chondroitin in metabolically engineered E. coli. Metab. Eng. 2015, 27, 92–100. [Google Scholar] [CrossRef]
- Cimini, D.; Iacono, I.D.; Carlino, E.; Finamore, R.; Restaino, O.F.; Diana, P.; Bedini, E.; Schiraldi, C. Engineering S. equi subsp. zooepidemicus towards concurrent production of hyaluronic acid and chondroitin biopolymers of biomedical interest. AMB Express 2017, 7, 61. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, R.; Chen, X.; Liu, L.; Xu, S.; Chen, J.; Wu, J. Enhancing fructosylated chondroitin production in Escherichia coli K4 by balancing the UDP-precursors. Metab. Eng. 2018, 47, 314–322. [Google Scholar] [CrossRef]
- Cheng, F.; Luozhong, S.; Yu, H.; Guo, Z. Biosynthesis of Chondroitin in Engineered Corynebacterium glutamicum. J. Microbiol. Biotechnol. 2019, 29, 392–400. [Google Scholar] [CrossRef]
- Kang, Z.; Zhou, Z.; Wang, Y.; Huang, H.; Du, G.; Chen, J. Bio-Based Strategies for Producing Glycosaminoglycans and Their Oligosaccharides. Trends Biotechnol. 2018, 36, 806–818. [Google Scholar] [CrossRef]
- Volpi, N. Quality of different chondroitin sulfate preparations in relation to their therapeutic activity. J. Pharm. Pharmacol. 2009, 61, 1271–1280. [Google Scholar] [CrossRef]
- Volpi, N. Analytical aspects of pharmaceutical grade chondroitin sulfates. J. Pharm. Sci. 2007, 96, 3168–3180. [Google Scholar] [CrossRef]
- Burgess, R.R. A brief practical review of size exclusion chromatography: Rules of thumb, limitations, and troubleshooting. Protein Expr. Purif. 2018, 150, 81–85. [Google Scholar] [CrossRef]
- Gama, C.I.; Tully, S.E.; Sotogaku, N.; Clark, P.M.; Rawat, M.; Vaidehi, N.; Goddard, W.A., 3rd; Nishi, A.; Hsieh-Wilson, L.C. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol. 2006, 2, 467–473. [Google Scholar] [CrossRef]
- Bedini, E.; De Castro, C.; De Rosa, M.; Di Nola, A.; Iadonisi, A.; Restaino, O.F.; Schiraldi, C.; Parrilli, M. A microbiological-chemical strategy to produce chondroitin sulfate A,C. Angew. Chem. (Int. Ed. Engl.) 2011, 50, 6160–6163. [Google Scholar] [CrossRef]
- Badri, A.; Williams, A.; Xia, K.; Linhardt, R.J.; Koffas, M.A.G. Increased 3′-Phosphoadenosine-5′-phosphosulfate Levels in Engineered Escherichia coli Cell Lysate Facilitate the In Vitro Synthesis of Chondroitin Sulfate A. Biotechnol. J. 2019, 14, e1800436. [Google Scholar] [CrossRef]
- Sugahara, K.; Kitagawa, H. Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struct. Biol. 2000, 10, 518–527. [Google Scholar] [CrossRef]
- Silbert, J.E.; Sugumaran, G. Biosynthesis of chondroitin/dermatan sulfate. IUBMB Life 2002, 54, 177–186. [Google Scholar] [CrossRef]
- Prydz, K.; Dalen, K.T. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000, 113, 193–205. [Google Scholar] [CrossRef]
- Habuchi, O. Diversity and functions of glycosaminoglycan sulfotransferases. Biochim. Biophys. Acta 2000, 1474, 115–127. [Google Scholar] [CrossRef]
- Hiraoka, N.; Misra, A.; Belot, F.; Hindsgaul, O.; Fukuda, M. Molecular cloning and expression of two distinct human N-acetylgalactosamine 4-O-sulfotransferases that transfer sulfate to GalNAc beta 1-->4GlcNAc beta 1-->R in both N- and O-glycans. Glycobiology 2001, 11, 495–504. [Google Scholar] [CrossRef]
- Yamada, T.; Ohtake, S.; Sato, M.; Habuchi, O. Chondroitin 4-sulphotransferase-1 and chondroitin 6-sulphotransferase-1 are affected differently by uronic acid residues neighbouring the acceptor GalNAc residues. Biochem. J. 2004, 384, 567–575. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Shashkov, A.S.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Structural characterization of fucosylated chondroitin sulfates from sea cucumbers Apostichopus japonicus and Actinopyga mauritiana. Carbohydr. Polym. 2016, 153, 399–405. [Google Scholar] [CrossRef]
- Guan, R.; Peng, Y.; Zhou, L.; Zheng, W.; Liu, X.; Wang, P.; Yuan, Q.; Gao, N.; Zhao, L.; Zhao, J. Precise Structure and Anticoagulant Activity of Fucosylated Glycosaminoglycan from Apostichopus japonicus: Analysis of Its Depolymerized Fragments. Mar. Drugs 2019, 17, 195. [Google Scholar] [CrossRef]
- Mou, J.; Li, Q.; Qi, X.; Yang, J. Structural comparison, antioxidant and anti-inflammatory properties of fucosylated chondroitin sulfate of three edible sea cucumbers. Carbohydr. Polym. 2018, 185, 41–47. [Google Scholar] [CrossRef]
- Grøndahl, F.; Tveit, H.; Akslen-Hoel, L.K.; Prydz, K. Easy HPLC-based separation and quantitation of chondroitin sulphate and hyaluronan disaccharides after chondroitinase ABC treatment. Carbohydr. Res. 2011, 346, 50–57. [Google Scholar] [CrossRef]
- Chen, S.-T.; Her, G.-R. Structural analysis of isomeric chondroitin sulfate oligosaccharides using regioselective 6-O-desulfation method and tandem mass spectrometry. Anal. Chim. Acta 2014, 843, 27–37. [Google Scholar] [CrossRef]
- Li, Q.; Cai, C.; Chang, Y.; Zhang, F.; Linhardt, R.J.; Xue, C.; Li, G.; Yu, G. A novel structural fucosylated chondroitin sulfate from Holothuria Mexicana and its effects on growth factors binding and anticoagulation. Carbohydr. Polym. 2018, 181, 1160–1168. [Google Scholar] [CrossRef]
- Millane, R.P.; Mitra, A.K.; Arnott, S. Chondroitin 4-sulfate: Comparison of the structures of the potassium and sodium salts. J. Mol. Biol. 1983, 169, 903–920. [Google Scholar] [CrossRef]
- Balt, S.; de Bolster, M.W.; Booij, M.; van Herk, A.M.; Visser-Luirink, G. Binding of metal ions to polysaccharides. V. Potentiometric, spectroscopic, and viscosimetric studies of the binding of cations to chondroitin sulfate and chondroitin in neutral and acidic aqueous media. J. Inorg. Biochem. 1983, 19, 213–226. [Google Scholar] [CrossRef]
- Ajisaka, K.; Oyanagi, Y.; Miyazaki, T.; Suzuki, Y. Effect of the chelation of metal cation on the antioxidant activity of chondroitin sulfates. Biosci. Biotechnol. Biochem. 2016, 80, 1179–1185. [Google Scholar] [CrossRef]
- Tanaka, K. Physicochemical properties of chondroitin sulfate. I. Ion binding and secondary structure. J. Biochem. 1978, 83, 647–653. [Google Scholar] [CrossRef]
- Parrish, R.F.; Fair, W.R. Selective binding of zinc ions to heparin rather than to other glycosaminoglycans. Biochem. J. 1981, 193, 407–410. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Bowness, J.M. Interaction of proteoglycans and chondroitin sulfates with calcium or phosphate ions. Can. J. Biochem. 1971, 49, 417–425. [Google Scholar] [CrossRef]
- Hunter, G.K.; Wong, K.S.; Kim, J.J. Binding of calcium to glycosaminoglycans: An equilibrium dialysis study. Arch. Biochem. Biophys. 1988, 260, 161–167. [Google Scholar] [CrossRef]
- Embery, G.; Rees, S.; Hall, R.; Rose, K.; Waddington, R.; Shellis, P. Calcium- and hydroxyapatite-binding properties of glucuronic acid-rich and iduronic acid-rich glycosaminoglycans and proteoglycans. Eur. J. Oral Sci. 1998, 106 (Suppl. S1), 267–273. [Google Scholar] [CrossRef]
- Guvench, O.; Whitmore, E.K. Sulfation and Calcium Favor Compact Conformations of Chondroitin in Aqueous Solutions. ACS Omega 2021, 6, 13204–13217. [Google Scholar] [CrossRef]
- Faller, C.E.; Guvench, O. Sulfation and Cation Effects on the Conformational Properties of the Glycan Backbone of Chondroitin Sulfate Disaccharides. J. Phys. Chem. B 2015, 119, 6063–6073. [Google Scholar] [CrossRef]
- Uchisawa, H.; Okuzaki, B.-i.; Ichita, J.; Matsue, H. Binding between calcium ions and chondroitin sulfate chains of salmon nasal cartilage glycosaminoglycan. Int. Congr. Ser. 2001, 1223, 205–220. [Google Scholar] [CrossRef]
- Cho, H.J.; Oh, J.; Choo, M.K.; Ha, J.I.; Park, Y.; Maeng, H.J. Chondroitin sulfate-capped gold nanoparticles for the oral delivery of insulin. Int. J. Biol. Macromol. 2014, 63, 15–20. [Google Scholar] [CrossRef]
- Xi, J.; Zhou, L.; Fei, Y. Preparation of chondroitin sulfate nanocapsules for use as carries by the interfacial polymerization method. Int. J. Biol. Macromol. 2012, 50, 157–163. [Google Scholar] [CrossRef]
- Zhang, J.S.; Imai, T.; Otagiri, M. Effects of a cisplatin-chondroitin sulfate A complex in reducing the nephrotoxicity of cisplatin. Arch. Toxicol. 2000, 74, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Higashi, K.; Ito, D.; Nagano, K.; Ishikawa, R.; Terui, Y.; Higashi, K.; Moribe, K.; Linhardt, R.J.; Toida, T. Poly-ion Complex of Chondroitin Sulfate and Spermine and Its Effect on Oral Chondroitin Sulfate Bioavailability. Chem. Pharm. Bull. 2016, 64, 390–398. [Google Scholar] [CrossRef]
- Sharma, S.; Swetha, K.L.; Roy, A. Chitosan-Chondroitin sulfate based polyelectrolyte complex for effective management of chronic wounds. Int. J. Biol. Macromol. 2019, 132, 97–108. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Krekic, S.; Miller, R.L.; Mero, M.; Pagel, K.; Heiner, Z. The interaction of chondroitin sulfate with a lipid monolayer observed by using nonlinear vibrational spectroscopy. Phys. Chem. Chem. Phys. PCCP 2021, 23, 13389–13395. [Google Scholar] [CrossRef]
- Rodrigues, S.A.; Pradal, C.; Yu, L.; Steadman, K.J.; Stokes, J.R.; Yakubov, G.E. Creating polysaccharide-protein complexes to control aqueous lubrication. Food Hydrocoll. 2021, 119, 106826. [Google Scholar] [CrossRef]
- Shi, X.-D.; Huang, J.-J.; Wu, J.-L.; Cai, X.-X.; Tian, Y.-Q.; Rao, P.-F.; Huang, J.-L.; Wang, S.-Y. Fabrication, interaction mechanism, functional properties, and applications of fish gelatin-polysaccharide composites: A review. Food Hydrocoll. 2022, 122, 107106. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Morell, P.; Nicoletti, V.R.; Quiles, A.; Hernando, I. Protein- and polysaccharide-based particles used for Pickering emulsion stabilisation. Food Hydrocoll. 2021, 119, 106839. [Google Scholar] [CrossRef]
- Quan, J.; Kim, S.-M.; Pan, C.-H.; Chung, D. Characterization of fucoxanthin-loaded microspheres composed of cetyl palmitate-based solid lipid core and fish gelatin–gum arabic coacervate shell. Food Res. Int. 2013, 50, 31–37. [Google Scholar] [CrossRef]
- Razzak, M.A.; Kim, M.; Chung, D. Elucidation of aqueous interactions between fish gelatin and sodium alginate. Carbohydr. Polym. 2016, 148, 181–188. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Liu, J. Development of active packaging based on chitosan-gelatin blend films functionalized with Chinese hawthorn (Crataegus pinnatifida) fruit extract. Int. J. Biol. Macromol. 2019, 140, 384–392. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, G.; Huang, H. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int. J. Biol. Macromol. 2019, 126, 842–845. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Wen, P.; Hong, Y.; Liu, X.; Xie, J. Preparation, characterization, antioxidant activity and protective effect against cellular oxidative stress of phosphorylated polysaccharide from Cyclocarya paliurus. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 145, 111754. [Google Scholar] [CrossRef]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef]
- Raman, R.; Sasisekharan, V.; Sasisekharan, R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem. Biol. 2005, 12, 267–277. [Google Scholar] [CrossRef]
- Properzi, F.; Carulli, D.; Asher, R.A.; Muir, E.; Camargo, L.M.; van Kuppevelt, T.H.; ten Dam, G.B.; Furukawa, Y.; Mikami, T.; Sugahara, K.; et al. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur. J. Neurosci. 2005, 21, 378–390. [Google Scholar] [CrossRef]
- Kitagawa, H.; Tsutsumi, K.; Tone, Y.; Sugahara, K. Developmental Regulation of the Sulfation Profile of Chondroitin Sulfate Chains in the Chicken Embryo Brain. J. Biol. Chem. 1997, 272, 31377–31381. [Google Scholar] [CrossRef]
- Mitsunaga, C.; Mikami, T.; Mizumoto, S.; Fukuda, J.; Sugahara, K. Chondroitin Sulfate/Dermatan Sulfate Hybrid Chains in the Development of Cerebellum: Spatiotemporal regulation of the expression of critical disulfated disaccharides by specific sulfotransferases. J. Biol. Chem. 2006, 281, 18942–18952. [Google Scholar] [CrossRef]
- Vigetti, D.; Andrini, O.; Clerici, M.; Negrini, D.; Passi, A.; Moriondo, A. Chondroitin sulfates act as extracellular gating modifiers on voltage-dependent ion channels. Cell. Physiol. Biochem. 2008, 22, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Uyama, T.; Ishida, M.; Izumikawa, T.; Trybala, E.; Tufaro, F.; Bergström, T.; Sugahara, K.; Kitagawa, H. Chondroitin 4-O-Sulfotransferase-1 Regulates E Disaccharide Expression of Chondroitin Sulfate Required for Herpes Simplex virus Infectivity. J. Biol. Chem. 2006, 281, 38668–38674. [Google Scholar] [CrossRef] [PubMed]
- Gowda, D.C. Role of Chondroitin-4-Sulfate in Pregnancy-Associated Malaria. In Advances in Pharmacology; Academic Press: Cambridge, MA, USA, 2006; Volume 53, pp. 375–400. [Google Scholar]
- Bergefall, K.; Trybala, E.; Johansson, M.; Uyama, T.; Naito, S.; Yamada, S.; Kitagawa, H.; Sugahara, K.; Bergström, T. Chondroitin sulfate characterized by the E-disaccharide unit is a potent inhibitor of herpes simplex virus infectivity and provides the virus binding sites on gro2C cells. J. Biol. Chem. 2005, 280, 32193–32199. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Sugahara, K.; Faissner, A. Chondroitin Sulfate “Wobble Motifs” Modulate Maintenance and Differentiation of Neural Stem Cells and Their Progeny. J. Biol. Chem. 2012, 287, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Mikami, T. Chondroitin/dermatan sulfate in the central nervous system. Curr. Opin. Struct. Biol. 2007, 17, 536–545. [Google Scholar] [CrossRef]
- Pantazaka, E.; Papadimitriou, E. Chondroitin sulfate-cell membrane effectors as regulators of growth factor-mediated vascular and cancer cell migration. Biochim. Biophys. Acta 2014, 1840, 2643–2650. [Google Scholar] [CrossRef]
- Mizumoto, S.; Yamada, S.; Sugahara, K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42. [Google Scholar] [CrossRef]
- Nadanaka, S.; Ishida, M.; Ikegami, M.; Kitagawa, H. Chondroitin 4-O-Sulfotransferase-1 Modulates Wnt-3a Signaling through Control of E Disaccharide Expression of Chondroitin Sulfate. J. Biol. Chem. 2008, 283, 27333–27343. [Google Scholar] [CrossRef]
- Nadanaka, S.; Kinouchi, H.; Taniguchi-Morita, K.; Tamura, J.-I.; Kitagawa, H. Down-regulation of Chondroitin 4-O-Sulfotransferase-1 by Wnt Signaling Triggers Diffusion of Wnt-3a. J. Biol. Chem. 2011, 286, 4199–4208. [Google Scholar] [CrossRef]
- Mikami, T.; Yasunaga, D.; Kitagawa, H. Contactin-1 Is a Functional Receptor for Neuroregulatory Chondroitin Sulfate-E. J. Biol. Chem. 2009, 284, 4494–4499. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Izumikawa, T.; Tamura, J.-i.; Kitagawa, H. Chondroitin sulfate-E fine-tunes osteoblast differentiation via ERK1/2, Smad3 and Smad1/5/8 signaling by binding to N-cadherin and cadherin-11. Biochem. Biophys. Res. Commun. 2012, 420, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Miyauchi, S.; Tawada, A.; Anada, T.; Matsuzaka, S.; Suzuki, O. Oversulfated chondroitin sulfate-E binds to BMP-4 and enhances osteoblast differentiation. J. Cell. Physiol. 2008, 217, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Uebelhart, D.; Malaise, M.; Marcolongo, R.; de Vathaire, F.; Piperno, M.; Mailleux, E.; Fioravanti, A.; Matoso, L.; Vignon, E. Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: A one-year, randomized, double-blind, multicenter study versus placebo. Osteoarthr. Cartil. 2004, 12, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, G.; Goemaere, S.; Veys, E.M. Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin. Rheumatol. 2002, 21, 231–243. [Google Scholar] [CrossRef]
- du Souich, P. Absorption, distribution and mechanism of action of SYSADOAS. Pharmacol. Ther. 2014, 142, 362–374. [Google Scholar] [CrossRef]
- Legendre, F.; Baugé, C.; Roche, R.; Saurel, A.S.; Pujol, J.P. Chondroitin sulfate modulation of matrix and inflammatory gene expression in IL-1beta-stimulated chondrocytes-study in hypoxic alginate bead cultures. Osteoarthr. Cartil. 2008, 16, 105–114. [Google Scholar] [CrossRef]
- Sakai, T.; Kyogashima, M.; Kariya, Y.; Urano, T.; Takada, Y.; Takada, A. Importance of GlcUAβ1-3GalNAc(4S,6S) in chondroitin sulfate E for t-PA- and u-PA-mediated Glu-plasminogen activation. Thromb. Res. 2000, 100, 557–565. [Google Scholar] [CrossRef]
- Maruyama, T.; Toida, T.; Imanari, T.; Yu, G.; Linhardt, R.J. Conformational changes and anticoagulant activity of chondroitin sulfate following its O-sulfonation. Carbohydr. Res. 1998, 306, 35–43. [Google Scholar] [CrossRef]
- Pudełko, A.; Wisowski, G.; Olczyk, K.; Koźma, E.M. The dual role of the glycosaminoglycan chondroitin-6-sulfate in the development, progression and metastasis of cancer. FEBS J. 2019, 286, 1815–1837. [Google Scholar] [CrossRef]
- Moto, M.; Takamizawa, N.; Shibuya, T.; Nakamura, A.; Katsuraya, K.; Iwasaki, K.; Tanaka, K.; Murota, A. Anti-diabetic effects of chondroitin sulfate on normal and type 2 diabetic mice. J. Funct. Foods 2018, 40, 336–340. [Google Scholar] [CrossRef]
- Ju, C.; Gao, J.; Hou, L.; Wang, L.; Zhang, F.; Sun, F.; Zhang, T.; Xu, P.; Shi, Z.; Hu, F.; et al. Neuroprotective effect of chondroitin sulfate on SH-SY5Y cells overexpressing wild-type or A53T mutant α-synuclein. Mol. Med. Rep. 2017, 16, 8721–8728. [Google Scholar] [CrossRef]
- Concha, M.; Vidal, A.; Giacaman, A.; Ojeda, J.; Pavicic, F.; Oyarzun-Ampuero, F.A.; Torres, C.; Cabrera, M.; Moreno-Villoslada, I.; Orellana, S.L. Aerogels made of chitosan and chondroitin sulfate at high degree of neutralization: Biological properties toward wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Wolff, R.B. Glucosamine and chondroitin sulfate association increases tibial epiphyseal growth plate proliferation and bone formation in ovariectomized rats. Clinics 2014, 69, 847–853. [Google Scholar] [CrossRef]
- Janssen, D.A.; van Wijk, X.M.; Jansen, K.C.; van Kuppevelt, T.H.; Heesakkers, J.P.; Schalken, J.A. The distribution and function of chondroitin sulfate and other sulfated glycosaminoglycans in the human bladder and their contribution to the protective bladder barrier. J. Urol. 2013, 189, 336–342. [Google Scholar] [CrossRef]
- Adebowale, A.O.; Cox, D.S.; Liang, Z.; Eddington, N.D. Analysis of glucosamine and chondroitin sulfate content in marketed products and the caco-2 permeability of chondroitin sulfate raw materials. J. Am. Nutraceutical Assoc. 2000, 3, 37–44. [Google Scholar]
- Zegels, B.; Crozes, P.; Uebelhart, D.; Bruyère, O.; Reginster, J.Y. Equivalence of a single dose (1200 mg) compared to a three-time a day dose (400 mg) of chondroitin 4&6 sulfate in patients with knee osteoarthritis. Results of a randomized double blind placebo controlled study. Osteoarthr. Cartil. 2013, 21, 22–27. [Google Scholar] [CrossRef]
- Henrotin, Y.; Marty, M.; Mobasheri, A. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas 2014, 78, 184–187. [Google Scholar] [CrossRef]
- Singh, J.A.; Noorbaloochi, S.; MacDonald, R.; Maxwell, L.J. Chondroitin for osteoarthritis. Cochrane Database Syst. Rev. 2015, 2016, CD005614. [Google Scholar] [CrossRef]
- Michel, B.A.; Stucki, G.; Frey, D.; De Vathaire, F.; Vignon, E.; Bruehlmann, P.; Uebelhart, D. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: A randomized, controlled trial. Arthritis Rheum. 2005, 52, 779–786. [Google Scholar] [CrossRef]
- Monfort, J.; Pelletier, J.P.; Garcia-Giralt, N.; Martel-Pelletier, J. Biochemical basis of the effect of chondroitin sulphate on osteoarthritis articular tissues. Ann. Rheum. Dis. 2008, 67, 735–740. [Google Scholar] [CrossRef]
- Uebelhart, D. Clinical review of chondroitin sulfate in osteoarthritis. Osteoarthr. Cartil. 2008, 16, S19–S21. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Wolfe, R.; Mai, T.; Lewis, D. A randomized, double blind, placebo controlled trial of a topical cream containing glucosamine sulfate, chondroitin sulfate, and camphor for osteoarthritis of the knee. J. Rheumatol. 2003, 30, 523–528. [Google Scholar]
- Pérez-Balbuena, A.L.; Ochoa-Tabares, J.C.; Belalcazar-Rey, S.; Urzúa-Salinas, C.; Saucedo-Rodríguez, L.R.; Velasco-Ramos, R.; Suárez-Sánchez, R.G.; Rodríguez-Carrizalez, A.D.; Oregón-Miranda, A.A. Efficacy of a fixed combination of 0.09 % xanthan gum/0.1 % chondroitin sulfate preservative free vs. polyethylene glycol/propylene glycol in subjects with dry eye disease: A multicenter randomized controlled trial. BMC Ophthalmol. 2016, 16, 164. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Majumdar, S.; Ma, G.; Sohn, J.; Yiu, S.C.; Stark, W.; Al-Qarni, A.; Edward, D.P.; Elisseeff, J.H. Chondroitin Sulfate-Based Biocompatible Crosslinker Restores Corneal Mechanics and Collagen Alignment. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3887–3895. [Google Scholar] [CrossRef]
- Vigan, M. Allergic contact dermatitis caused by sodium chondroitin sulfate contained in a cosmetic cream. Contact Dermat. 2014, 70, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Bensi, D.; Caglio, G. Compositions Comprising Dermatan Sulfate and Chondroitin Sulfate and Use Thereof in Cosmetological Compositions. AU Patent AU20160304201, 16 March 2016. [Google Scholar]
- Min, D.; Park, S.; Kim, H.; Lee, S.H.; Ahn, Y.; Jung, W.; Kim, H.J.; Cho, Y.W. Potential anti-ageing effect of chondroitin sulphate through skin regeneration. Int. J. Cosmet. Sci. 2020, 42, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Torella, M.; Del Deo, F.; Grimaldi, A.; Iervolino, S.A.; Pezzella, M.; Tammaro, C.; Gallo, P.; Rappa, C.; De Franciscis, P.; Colacurci, N. Efficacy of an orally administered combination of hyaluronic acid, chondroitin sulfate, curcumin and quercetin for the prevention of recurrent urinary tract infections in postmenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 125–128. [Google Scholar] [CrossRef]
- Imperatore, V.; Creta, M.; Di Meo, S.; Buonopane, R.; Longo, N.; Fusco, F.; Spirito, L.; Imbimbo, C.; Mirone, V. Intravesical administration of combined hyaluronic acid and chondroitin sulfate can improve symptoms in patients with refractory bacillus Calmette-Guerin-induced chemical cystitis: Preliminary experience with one-year follow-up. Arch. Ital. Urol. Androl. 2018, 90, 11–14. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Rikkers, M.; Mihajlovic, M.; Viola, M.; Schuiringa, G.; Ilochonwu, B.C.; Masereeuw, R.; Vonk, L.; Malda, J.; Ito, K.; et al. Viscoelastic Chondroitin Sulfate and Hyaluronic Acid Double-Network Hydrogels with Reversible Cross-Links. Biomacromolecules 2022, 23, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111324. [Google Scholar] [CrossRef] [PubMed]
- Schuurmans, C.C.L.; Mihajlovic, M.; Hiemstra, C.; Ito, K.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and chondroitin sulfate (meth)acrylate-based hydrogels for tissue engineering: Synthesis, characteristics and pre-clinical evaluation. Biomaterials 2021, 268, 120602. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, Y.; Adoungotchodo, A.; Hui, E.; Zehtabi, F.; Lerouge, S. An injectable chitosan/chondroitin sulfate hydrogel with tunable mechanical properties for cell therapy/tissue engineering. Int. J. Biol. Macromol. 2018, 113, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Jung, U.W.; Noh, I. Synthesis and Biocompatibility Characterizations of in Situ Chondroitin Sulfate-Gelatin Hydrogel for Tissue Engineering. Tissue Eng. Regen. Med. 2018, 15, 25–35. [Google Scholar] [CrossRef]
- Kim, H.D.; Lee, E.A.; An, Y.H.; Kim, S.L.; Lee, S.S.; Yu, S.J.; Jang, H.L.; Nam, K.T.; Im, S.G.; Hwang, N.S. Chondroitin Sulfate-Based Biomineralizing Surface Hydrogels for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 21639–21650. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, M.; Ari, B.; Sunol, A.K.; Sahiner, N. Chondroitin Sulfate-Based Cryogels for Biomedical Applications. Gels 2021, 7, 127. [Google Scholar] [CrossRef]
- Mercuri, J.J.; Gill, S.S.; Simionescu, D.T. Novel tissue-derived biomimetic scaffold for regenerating the human nucleus pulposus. J. Biomed. Mater. Res. Part A 2011, 96, 422–435. [Google Scholar] [CrossRef]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Nanomed. 2012, 7, 1101–1114. [Google Scholar] [CrossRef]
- Caliari, S.R.; Harley, B.A.C. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef]
- Kavya, K.C.; Dixit, R.; Jayakumar, R.; Nair, S.V.; Chennazhi, K.P. Synthesis and characterization of chitosan/chondroitin sulfate/nano-SiO2 composite scaffold for bone tissue engineering. J. Biomed. Nanotechnol. 2012, 8, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Yan, D. An Injectable Enzymatically Crosslinked Carboxymethylated Pullulan/Chondroitin Sulfate Hydrogel for Cartilage Tissue Engineering. Sci. Rep. 2016, 6, 20014. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Sinha, S.; Rastogi, A.; Srivastava, P. Generation of scaffold incorporated with nanobioglass encapsulated in chitosan/chondroitin sulfate complex for bone tissue engineering. Int. J. Biol. Macromol. 2020, 153, 1–16. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Lee, M.S.; Zhang, Z.; Li, B.; Xuan, H.; Li, W.J.; Zhang, Y. Collagen and chondroitin sulfate functionalized bioinspired fibers for tendon tissue engineering application. Int. J. Biol. Macromol. 2021, 170, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, X.; Cai, D.; Li, J.; Mu, Q.; Zhang, W.; Zhu, S.; Jiang, Y.; Shen, W.; Zhang, S.; et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017, 63, 64–75. [Google Scholar] [CrossRef]
- Bhowmick, S.; Rother, S.; Zimmermann, H.; Lee, P.S.; Moeller, S.; Schnabelrauch, M.; Koul, V.; Jordan, R.; Hintze, V.; Scharnweber, D. Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application—The role of chondroitin sulfate and sulfated hyaluronan. Mater. Sci. Eng. C 2017, 79, 15–22. [Google Scholar] [CrossRef]
- Sadeghi, A.; Zandi, M.; Pezeshki-Modaress, M.; Rajabi, S. Tough, hybrid chondroitin sulfate nanofibers as a promising scaffold for skin tissue engineering. Int. J. Biol. Macromol. 2019, 132, 63–75. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Malavasi, L.; Fante, C.D.; Vigani, B.; Black, L.; Ferrari, F. Electrospun Gelatin–Chondroitin Sulfate Scaffolds Loaded with Platelet Lysate Promote Immature Cardiomyocyte Proliferation. Polymers 2018, 10, 208. [Google Scholar] [CrossRef]
- Chen, S.; Chen, W.; Chen, Y.; Mo, X.; Fan, C. Chondroitin sulfate modified 3D porous electrospun nanofiber scaffolds promote cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111312. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Z.; Copland, J.A.; Chakrabarti, R.; Florczyk, S.J. 3D porous chitosan-chondroitin sulfate scaffolds promote epithelial to mesenchymal transition in prostate cancer cells. Biomaterials 2020, 254, 120126. [Google Scholar] [CrossRef]
- Huang, X.; Xu, C.; Li, Y.; Cheng, H.; Wang, X.; Sun, R. Quaternized chitosan-stabilized copper sulfide nanoparticles for cancer therapy. Mater. Sci. Eng. C 2019, 96, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ling, Y.; Cao, C.; Li, X.; Chen, X.; Wang, X. Chitosan derivatives/reduced graphene oxide/alginate beads for small-molecule drug delivery. Mater. Sci. Eng. C 2016, 69, 1222–1228. [Google Scholar] [CrossRef]
- Shao, P.; Xuan, S.; Wu, W.; Qu, L. Encapsulation efficiency and controlled release of Ganoderma lucidum polysaccharide microcapsules by spray drying using different combinations of wall materials. Int. J. Biol. Macromol. 2019, 125, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Amhare, A.F.; Lei, J.; Deng, H.; Lv, Y.; Han, J.; Zhang, L. Biomedical application of chondroitin sulfate with nanoparticles in drug delivery systems: Systematic review. J. Drug Target. 2021, 29, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zhang, Z.; Jing, P. Black rice anthocyanins embedded in self-assembled chitosan/chondroitin sulfate nanoparticles enhance apoptosis in HCT-116 cells. Food Chem. 2019, 301, 125280. [Google Scholar] [CrossRef]
- Zu, M.; Ma, L.; Zhang, X.; Xie, D.; Kang, Y.; Xiao, B. Chondroitin sulfate-functionalized polymeric nanoparticles for colon cancer-targeted chemotherapy. Colloids Surf. B Biointerfaces 2019, 177, 399–406. [Google Scholar] [CrossRef]
- Morath, I.; Hartmann, T.N.; Orian-Rousseau, V. CD44: More than a mere stem cell marker. Int. J. Biochem. Cell Biol. 2016, 81, 166–173. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.; Du, X.; Yang, H.; Liu, Y.; Khan, A.Q.; Zhai, G. Chondroitin sulfate derived theranostic and therapeutic nanocarriers for tumor-targeted drug delivery. Carbohydr. Polym. 2020, 233, 115837. [Google Scholar] [CrossRef]
- Liu, P.; Chen, N.; Yan, L.; Gao, F.; Ji, D.; Zhang, S.; Zhang, L.; Li, Y.; Xiao, Y. Preparation, characterisation and in vitro and in vivo evaluation of CD44-targeted chondroitin sulphate-conjugated doxorubicin PLGA nanoparticles. Carbohydr. Polym. 2019, 213, 17–26. [Google Scholar] [CrossRef]
- Fernandez-Piñeiro, I.; Pensado, A.; Badiola, I.; Sanchez, A. Development and characterisation of chondroitin sulfate- and hyaluronic acid-incorporated sorbitan ester nanoparticles as gene delivery systems. Eur. J. Pharm. Biopharm. 2018, 125, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Liang, X.; Huang, Y.; Li, Q. Chondroitin sulfate-functionalized polyamidoamine as a tumor-targeted carrier for miR-34a delivery. Acta Biomater. 2017, 57, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Okubo, M.; Miyazaki, M.; Yuba, E.; Harada, A. Chondroitin Sulfate-Based pH-Sensitive Polymer-Modified Liposomes for Intracellular Antigen Delivery and Induction of Cancer Immunity. Bioconjugate Chem. 2019, 30, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, B.; Kong, W.; Yuan, L.; Guo, L.; Li, C.; Fan, H.; Fan, Y.; Zhang, X. Injectable and self-crosslinkable hydrogels based on collagen type II and activated chondroitin sulfate for cell delivery. Int. J. Biol. Macromol. 2018, 118, 2014–2020. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Fang, W.; Tao, Y.; Zhao, T.; Xia, K.; Liang, C.; Hua, J.; Li, F.; Chen, Q. Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018, 71, 496–509. [Google Scholar] [CrossRef]
- Kashiuchi, S.; Miyazawa, R.; Nagata, H.; Shirai, M.; Shimizu, M.; Sone, H.; Kamiyama, S. Effects of administration of glucosamine and chicken cartilage hydrolysate on rheumatoid arthritis in SKG mice. Food Funct. 2019, 10, 5008–5017. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, C.; Qin, X.; Zhang, H.; Zhang, Z.; Richel, A. Modulation of gut microbiota by chondroitin sulfate calcium complex during alleviation of osteoporosis in ovariectomized rats. Carbohydr. Polym. 2021, 266, 118099. [Google Scholar] [CrossRef]
- Han, L.K.; Sumiyoshi, M.; Takeda, T.; Chihara, H.; Nishikiori, T.; Tsujita, T.; Kimura, Y.; Okuda, H. Inhibitory effects of chondroitin sulfate prepared from salmon nasal cartilage on fat storage in mice fed a high-fat diet. Int. J. Obes. 2000, 24, 1131–1138. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Mao, G.; Hu, Y.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. Fucosylated chondroitin sulfate oligosaccharides from Isostichopus badionotus regulates lipid disorder in C57BL/6 mice fed a high-fat diet. Carbohydr. Polym. 2018, 201, 634–642. [Google Scholar] [CrossRef]
- Wu, R.; Shang, N.; Gui, M.; Yin, J.; Li, P. Sturgeon (Acipenser)-Derived Chondroitin Sulfate Suppresses Human Colon Cancer HCT-116 Both In Vitro and In Vivo by Inhibiting Proliferation and Inducing Apoptosis. Nutrients 2020, 12, 1130. [Google Scholar] [CrossRef]
- Rani, A.; Baruah, R.; Goyal, A. Prebiotic Chondroitin Sulfate Disaccharide Isolated from Chicken Keel Bone Exhibiting Anticancer Potential against Human Colon Cancer Cells. Nutr. Cancer 2019, 71, 825–839. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Soroush, A.R.; Angoorani, P.; Larijani, B.; Hasani-Ranjbar, S. Gut Microbiota as a Target in the Pathogenesis of Metabolic Disorders: A New Approach to Novel Therapeutic Agents. Horm. Metab. Res. 2016, 48, 349–358. [Google Scholar] [CrossRef]
- Shang, Q.; Yin, Y.; Zhu, L.; Li, G.; Yu, G.; Wang, X. Degradation of chondroitin sulfate by the gut microbiota of Chinese individuals. Int. J. Biol. Macromol. 2016, 86, 112–118. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, N.; Li, Z.; Wang, X.; Shi, H.; Xue, C.; Li, R.W.; Tang, Q. Chondroitin sulfate disaccharides modified the structure and function of the murine gut microbiome under healthy and stressed conditions. Sci. Rep. 2017, 7, 6783. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Mao, G.; Wu, T.; Lin, D.; Hu, Y.; Ye, X.; Tian, D.; Chai, W.; Linhardt, R.J.; et al. Fucosylated chondroitin sulfate from Isostichopus badionotus alleviates metabolic syndromes and gut microbiota dysbiosis induced by high-fat and high-fructose diet. Int. J. Biol. Macromol. 2019, 124, 377–388. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Xu, Y.; Yang, H.; Wang, J.; Xue, C.; Yan, X.; Su, L. Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice. Food Funct. 2019, 10, 1736–1746. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef]
- McCabe, L.; Britton, R.A.; Parameswaran, N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef]
- Weaver, C.M. Diet, gut microbiome, and bone health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]





| Strain Source | Process | Product | Yield (g/L) | Ref. |
|---|---|---|---|---|
| E. coli | Batch+P | K4 CPS | 0.08–0.09 | [84] |
| E. coli | Batch+P | K4 CPS | 0.2 | [85] |
| E. coli | Batch | K4 CPS | 0.42 | [97] |
| E. coli | Batch | K4 CPS | 0.3 | [98] |
| E. coli | ISPR | K4 CPS | 4.7 | [87] |
| E. coli | Fed-batch+P | Ch | 3 | [99] |
| E. coli | Batch | K4 CPS | 0.41 | [100] |
| B. subtilis BN | Batch | CS | 4.2 | [89] |
| B. natto | Shake flask | CS | 0.24 | [101] |
| E. coli K4+kfoC (E. coli) | Batch | K4 CPS+Ch | 0.48 | [102] |
| E. coli K4 (mutant kfoC) | Shake flask | K4 CPS+Ch | 0.21 | [103] |
| E. coli K4+rfaH (E. coli) | Fed-batch | K4 CPS+Ch | 5.3 | [104] |
| E. coli K4+slyA (E. coli) | Fed-batch | K4 CPS | 2.6 | [105] |
| E. coli K4+kfoC (E. coli) | Fed-batch | K4 CPS+Ch | 3.5 | [106] |
| E. coli K4 (ΔkfoE)+kfoE (E. coli) | Batch | Ch | 1.19 | [95] |
| E. coli K4 + pgm, galU, rfaH (E. coli) | Microbioreactor batch | K4 CPS | 0.59 | [107] |
| B. subtilis + tuaD (B. subtilis) | Fed-batch | Ch | 2.54 | [90] |
| E. coli BL21 + kfoA, kfoC, kfoF (E. coli) | Fed-batch | Ch | 2.4 | [108] |
| S. equi subsp. Zooepidemicus+ kfoA,kfoC (E. coli) | Bioreactor batch+P | Ch | 0.3 | [109] |
| B. subtilis + tuaD, glmM, kfoA (B. subtilis) | Fed-batch | Ch | 7.15 | [83] |
| E. coli K4 (ΔpfkA, mutant kfoC)+glmM,glmS,galU,pgm((E. coli) | DO-stat feeding batch | Fructosylated- Ch | 8.43 | [110] |
| C. glutamicum (Δldh)+ kfoC,kfoA (E. coli)+ ugdA(C. glutamicum) | Fed-batch | Ch | 1.91 | [111] |
| P. pastoris + kfoC,kfoA (E. coli)+ tuaD (B. subtilis) | Fed-batch | Ch | 0.19 | [11] |
| Bioactivity | Component | Biological Effects | Ref. |
|---|---|---|---|
| Anti-inflammation | CS | Repress the expression of genes encoding proteolytic enzymes; inhibit IL-1β-induced expression of the pro-inflammatory genes iNOS and COX-2 and restores TGF-β receptors I and II mRNA levels. | [180] |
| Anti-thrombus | CS-E | Enhances plasminogen activation. | [181] |
| Anti-coagulation | O-sulfonated CS | Increases anti-factor IIa activity and anti-factor Xa activity. | [182] |
| Anti-oxidation | CS and CS–metal complex | Enhance hydroxyl radical or superoxide radical scavenging activity. | [133] |
| Anti-tumor | CS-C | Influences tumor-associated inflammation; affects NF-κB signaling and cell behavior and regulates cytokine/chemokine activity. | [183] |
| Anti-viral | CS-E | Interferes with the binding of viral gC to a CS-E-like receptor on the cell surface. | [167] |
| Anti-diabetes | CS | Reduces the digestion of carbohydrates; reduces hyperglycemia. | [184] |
| Anti-obesity | CS | Ameliorates obesity; prevents the gaining of body weight, liver weight, and adipose tissue weight; maintains lower food consumption; inhibits the intestinal absorption of triglyceride; adjusts the serum endotoxin level. | [18] |
| Neuroprotective | CS sodium salt | Downregulates P-Ser129 α-synuclein and total α-synuclein expression; inhibits ROS overproduction and changes mitochondrion-mediated apoptotic pathways. | [185] |
| Wound healing | CS aerogel | High hydration and rapid setting to the wound bed. | [186] |
| Proliferation and bone formation | CS and Glucosamine | Proliferates chondrocytes; increases remaining cartilage and trabecula. | [187] |
| Protective bladder barrier | CS | Contributes to urothelial barrier function. | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Q.; Guo, Y.; Wang, K.; Zhang, C.; Ma, Y. A Review of Chondroitin Sulfate’s Preparation, Properties, Functions, and Applications. Molecules 2023, 28, 7093. https://doi.org/10.3390/molecules28207093
Shen Q, Guo Y, Wang K, Zhang C, Ma Y. A Review of Chondroitin Sulfate’s Preparation, Properties, Functions, and Applications. Molecules. 2023; 28(20):7093. https://doi.org/10.3390/molecules28207093
Chicago/Turabian StyleShen, Qingshan, Yujie Guo, Kangyu Wang, Chunhui Zhang, and Yanli Ma. 2023. "A Review of Chondroitin Sulfate’s Preparation, Properties, Functions, and Applications" Molecules 28, no. 20: 7093. https://doi.org/10.3390/molecules28207093
APA StyleShen, Q., Guo, Y., Wang, K., Zhang, C., & Ma, Y. (2023). A Review of Chondroitin Sulfate’s Preparation, Properties, Functions, and Applications. Molecules, 28(20), 7093. https://doi.org/10.3390/molecules28207093