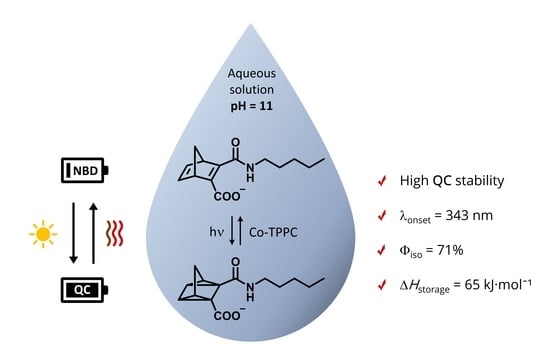
A Norbornadiene-Based Molecular System for the Storage of Solar–Thermal Energy in an Aqueous Solution: Study of the Heat-Release Process Triggered by a Co(II)-Complex
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acid Dissociation of HNBD1 and HQC1 and Solution Stability
2.2. Photochemical Properties
2.3. Back-Conversion of HQC1/QC1− to HNBD1/NBD1−
3. Materials and Methods
3.1. General
3.2. Synthesis of HNBD1 and HQC1
3.2.1. Synthesis of Diethyl Bicyclo[2.2.1]hepta-2,5-diene-2,3-dicarboxylate (1)
3.2.2. Synthesis of 3-(ethoxycarbonyl)bicyclo[2.2.1]hepta-2,5-diene-2-carboxylic acid (2)
3.2.3. Synthesis of Ethyl 3-(pentylcarbamoyl)bicyclo[2.2.1]hepta-2,5-diene-2-carboxylate (3)
3.2.4. Synthesis of 3-(pentylcarbamoyl)bicyclo[2.2.1]hepta-2,5-diene-2-carboxylic Acid (HNBD1)
3.2.5. Synthesis of 5-(pentylcarbamoyl)tetracyclo[3.2.0.02,7.04,6]heptane-1-carboxylic Acid (HQC1)
3.3. Physicochemical Properties of HNBD1/NBD1− and HQC1/QC1−
3.3.1. Spectrophotometric Measurements
3.3.2. Isothermal Titration Calorimetry (ITC)
3.3.3. Differential Scanning Calorimetry (DSC)
3.3.4. Nuclear Magnetic Resonance (NMR)
3.3.5. Photoisomerization Quantum Yield
3.3.6. Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Miljanic, O.S.; Pratt, J.A. Introduction to Energy and Sustainability; Wiley-VCH: Weinheim, Germany, 2021. [Google Scholar]
- Sheikholeslami, M. (Ed.) Nanotechnology Applications for Solar Energy Systems; John Wiley and Sons: New York, NY, USA, 2023. [Google Scholar]
- Wang, Z.; Hölzel, H.; Moth-Poulsen, K. Status and challenges for molecular solar thermal energy storage system based devices. Chem. Soc. Rev. 2022, 51, 7313–7326. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Gomez, A.; Magson, L.; Peñin, B.; Sanosa, N.; Soilán, J.; Losantos, R.; Sampedro, D. A Photochemical Overview of Molecular Solar Thermal Energy Storage. Photochem 2022, 2, 694–716. [Google Scholar] [CrossRef]
- Wang, Z.; Erhart, P.; Li, T.; Zhang, Z.-Y.; Sampedro, D.; Hu, Z.; Wegner, H.A.; Brummel, O.; Libuda, J.; Nielsen, M.B.; et al. Storing energy with molecular photoisomers. Joule 2021, 5, 3116–3136. [Google Scholar] [CrossRef]
- Qiu, Q.; Shi, Y.; Han, G.G.D. Solar energy conversion and storage by photoswitchable organic materials in solution, liquid, solid, and changing phases. J. Mater. Chem. C 2021, 9, 11444–11463. [Google Scholar] [CrossRef]
- Nielsen, M.B.; Ree, N.; Mikkelsen, K.V.; Cacciarini, M. Tuning the Dihydroazulene-Vinylheptafulvene Couple for Storage of Solar Energy. Russ. Chem. Rev. 2020, 89, 573–586. [Google Scholar] [CrossRef]
- Sun, C.-L.; Wang, C.; Boulatov, R. Applications of Photoswitches in the Storage of Solar Energy. ChemPhotoChem 2019, 3, 268–283. [Google Scholar] [CrossRef]
- Ganguly, G.; Sultana, M.; Paul, A. Designing Efficient Solar-Thermal Fuels with [n.n](9,10)Anthracene Cyclophanes: A Theoretical Perspective. J. Phys. Chem. Lett. 2018, 9, 328–334. [Google Scholar] [CrossRef]
- Dong, L.; Feng, Y.; Wang, L.; Feng, W. Azobenzene-based Solar Thermal Fuels: Design, Properties, and Applications. Chem. Soc. Rev. 2018, 47, 7339–7368. [Google Scholar] [CrossRef]
- Cacciarini, M.; Skov, A.B.; Jevric, M.; Hansen, A.S.; Elm, J.; Kjaergaard, H.G.; Mikkelsen, K.V.; Nielsen, M.B. Towards Solar Energy Storage in the Photochromic Dihydroazulene-Vinylheptafulvene System. Chem. Eur. J. 2015, 21, 7454–7461. [Google Scholar] [CrossRef]
- Krekiehn, N.R.; Müller, M.; Jung, U.; Ulrich, S.; Herges, R.; Magnussen, O.M. UV/Vis Spectroscopy Studies of the Photoisomerization Kinetics in Self-Assembled Azobenzene-Containing Adlayers. Langmuir 2015, 31, 8362–8370. [Google Scholar] [CrossRef]
- Lennartson, A.; Roffey, A.; Moth-Poulsen, K. Designing Photoswitches for Molecular Solar Thermal Energy Storage. Tetrahedron Lett. 2015, 56, 1457–1465. [Google Scholar] [CrossRef]
- Kucharski, T.J.; Ferralis, N.; Kolpak, A.M.; Zheng, J.O.; Nocera, D.G.; Grossman, J.C. Templated assembly of photoswitches significantly increases the energy-storage capacity of solar thermal fuels. Nat. Chem. 2014, 6, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, T.J.; Tian, Y.; Akbulatov, S.; Boulatov, R. Chemical solutions for the closed-cycle storage of solar energy. Energy Environ. Sci. 2011, 4, 4449–4472. [Google Scholar] [CrossRef]
- Kolpak, A.M.; Grossman, J.C. Azobenzene-functionalized Carbon Nanotubes as High-energy Density Solar Thermal Fuels. Nano Lett. 2011, 11, 3156–3162. [Google Scholar] [CrossRef]
- Bastianelli, C.; Caia, V.; Cum, G.; Gallo, R.; Mancini, V. Thermal Isomerization of Photochemically Synthesized (Z.)-9-styrylacridines. An Unusually High Enthalpy of Z → E Conversion for Stilbene-like Compounds. J. Chem. Soc. Perkin Trans. 1991, 2, 679–683. [Google Scholar] [CrossRef]
- Xing, X.; Gedanken, A.; Sheybani, A.-H.; McDiarmid, R. The 198-225 nm Transition of Norbornadiene. J. Phys. Chem. 1994, 98, 8302–8309. [Google Scholar] [CrossRef]
- Roos, B.O.; Merchan, M.; McDiarmid, R.; Xing, X. Theoretical and Experimental Determination of the Electronic Spectrum of Norbornadiene. J. Am. Chem. Soc. 1994, 116, 5927–5936. [Google Scholar] [CrossRef]
- Yoshida, Z.J. New molecular energy storage systems. J. Photochem. 1985, 29, 27–40. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Dreos, A.; Moth-Poulsen, K. Engineering of Norbornadiene/Quadricyclane Photoswitches for Molecular Solar Thermal Energy Storage Applications. Acc. Chem. Res. 2020, 53, 1478–1487. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Z.; Mu, E.; Zhang, Z.-Y.; Jevric, M.; Liu, Y.; Orrego-Hernández, J.; Wu, Z.; Fu, X.; Wang, F.; et al. Molecular Solar Thermal Power Generation. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Kashyap, V.; Sakunkaewkasem, S.; Jafari, P.; Nazari, M.; Eslami, B.; Nazifi, S.; Irajizad, P.; Marquez, M.D.; Lee, T.R.; Ghasemi, H. Full spectrum solar thermal energy harvesting and storage by a molecular and phase-change hybrid material. Joule 2019, 3, 3100–3111. [Google Scholar] [CrossRef]
- Wang, Z.; Roffey, A.; Losantos, R.; Lennartson, A.; Jevric, M.; Petersen, A.U.; Quant, M.; Dreos, A.; Wen, X.; Sampedro, D.; et al. Macroscopic heat release in a molecular solar thermal energy storage system. Energy Environ. Sci. 2019, 12, 187–193. [Google Scholar] [CrossRef]
- Dreos, A.; Börjesson, K.; Wang, Z.; Roffey, A.; Norwood, Z.; Kushnir, D.; Moth-Poulsen, K. Exploring the potential of a hybrid device combining solar water heating and molecular solar thermal energy storage. Energy Environ. Sci. 2017, 10, 728–734. [Google Scholar] [CrossRef]
- Yasufuku, K.; Takahashi, K.; Kutal, C. Electrochemical catalysis of the valence isomerization of quadricyclene. Tetrahedron Lett. 1984, 25, 4893–4896. [Google Scholar] [CrossRef]
- Gassman, P.G.; Hershberger, J.W. An electrochemical switch for starting and stopping the energy-releasing conversion of quadricyclanes to norbornadienes. J. Org. Chem. 1987, 52, 1337–1339. [Google Scholar] [CrossRef]
- Gray, V.; Lennartson, A.; Ratanalert, P.; Borjesson, K.; Moth-Poulsen, K. Diaryl-substituted norbornadienes with red-shifted absorption for molecular solar thermal energy storage. Chem. Commun. 2014, 50, 5330–5332. [Google Scholar] [CrossRef]
- Brummel, O.; Waidhas, F.; Bauer, U.; Wu, Y.; Bochmann, S.; Steinrück, H.-P.; Papp, C.; Bachmann, J.; Libuda, J. Photochemical Energy Storage and Electrochemically Triggered Energy Release in the Norbornadiene−Quadricyclane System: UV Photochemistry and IR Spectroelectrochemistry in a Combined Experiment. J. Phys. Chem. Lett. 2017, 8, 2819–2825. [Google Scholar] [CrossRef]
- Waidhas, F.; Jevric, M.; Bosch, M.; Yang, T.; Franz, E.; Liu, Z.; Bachmann, J.; Moth-Poulsen, K.; Brummel, O.; Libuda, J. Electrochemically controlled energy release from a norbornadiene-based solar thermal fuel: Increasing the reversibility to 99.8% using HOPG as the electrode material. J. Mater. Chem. A 2020, 8, 15658–15664. [Google Scholar] [CrossRef]
- Franz, E.; Krappmann, D.; Fromm, L.; Luchs, T.; Görling, A.; Hirsch, A.; Brummel, O.; Libuda, J. Electrocatalytic Energy Release of Norbornadiene-Based Molecular Solar Thermal Systems: Tuning the Electrochemical Stability by Molecular Design. ChemSusChem 2022, 15, e202201483. [Google Scholar] [CrossRef]
- Jones, G., II; Chiang, S.; Xuan, P.T. Energy storage in organic photoisomers. J. Photochem. 1979, 10, 1–18. [Google Scholar] [CrossRef]
- Maruyama, K.; Terada, K.; Yamamoto, Y. Exploitation of solar energy storage systems. Valence isomerization between norbornadiene and quadricyclane derivatives. J. Org. Chem. 1981, 46, 5294–5300. [Google Scholar] [CrossRef]
- Maruyama, K.; Tamiaki, H. Catalytic Isomerization of Water-Soluble Quadricyclane to Norbornadiene Derivatives Induced by Cobalt-Porphyrin Complexes. J. Org. Chem. 1986, 51, 602–606. [Google Scholar] [CrossRef]
- Maruyama, K.; Tamiaki, H.; Kawabata, S. Exothermic Isomerization of Water-soluble Quadricyclanes to Norbornadienes by Soluble and Insoluble Catalysts. J. Chem. Soc. Perkin Trans. II 1986, 543–549. [Google Scholar] [CrossRef]
- Eschenbacher, R.; Xu, T.; Franz, E.; Löw, R.; Moje, T.; Fromm, L.; Görling, A.; Brummel, O.; Herges, R.; Libuda, J. Triggering the energy release in molecular solar thermal systems: Norbornadiene-functionalized trioxatriangulen on Au(111). NanoEnergy 2022, 95, 10700. [Google Scholar] [CrossRef]
- Bauer, U.; Mohr, S.; Döpper, T.; Bachmann, P.; Späth, F.; Düll, F.; Schwarz, M.; Brummel, O.; Fromm, L.; Pinkert, U.; et al. Catalytically Triggered Energy Release from Strained Organic Molecules: The Surface Chemistry of Quadricyclane and Norbornadiene on Pt(111). Chemistry 2017, 23, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Bauer, U.; Fromm, L.; Weiß, C.; Bachmann, P.; Späth, F.; Düll, F.; Steinhauer, J.; Hieringer, W.; Görling, A.; Hirsch, A.; et al. Controlled Catalytic Energy Release of the Norbornadiene/Quadricyclane Molecular Solar Thermal Energy Storage System on Ni(111). J. Phys. Chem. C 2019, 123, 7654–7664. [Google Scholar] [CrossRef]
- Bauer, U.; Fromm, L.; Weiß, C.; Späth, F.; Bachmann, P.; Düll, F.; Steinhauer, J.; Matysik, S.; Pominov, A.; Görling, A.; et al. Surface chemistry of 2,3-dibromosubstituted norbornadiene/quadricyclane as molecular solar thermal energy storage system on Ni(111). J. Chem. Phys. 2019, 150, 184706. [Google Scholar] [CrossRef]
- Lorenz, P.; Luchs, T.; Hirsch, A. Molecular Solar Thermal Batteries through Combination of Magnetic Nanoparticle Catalysts and Tailored Norbornadiene Photoswitches. Chem. Eur. J. 2021, 27, 4993–5002. [Google Scholar] [CrossRef]
- Miki, S.; Maruyama, T.; Ohno, T.; Tohma, T.; Toyama, S.; Yoshida, Z. Alumina-anchored Cobalt(II) Schiff Base Catalyst for the Isomerization of Trimethyldicyanoquadricyclane to the Norbornadiene. Chem. Lett. 1988, 17, 861–864. [Google Scholar] [CrossRef]
- Miki, S.; Asako, Y.; Morimoto, M.; Ohno, T.; Yoshida, Z.; Maruyama, T.; Fukuoka, M.; Takada, T. Alumina-Anchored Cobalt Porphine Catalysts for the Conversion of Quadricyclane to Norbornadiene. Bull. Chem. Soc. Jpn. 1988, 61, 973–981. [Google Scholar] [CrossRef]
- Tchougreeff, A.L.; Tokmachev, A.M.; Dronskowski, R.R. Resonance theory of catalytic action of transition-metal complexes: Isomerization of quadricyclane to norbornadiene catalyzed by metal porphyrins. Int. J. Quantum Chem. 2013, 113, 1833–1846. [Google Scholar] [CrossRef]
- Bren’, V.A.; Dubonosov, A.D.; Minkin, V.I.; Chernoivanov, V.A. Norbornadiene–quadricyclane—An effective molecular system for the storage of solar energy. Russ. Chem. Rev. 1991, 60, 451–469. [Google Scholar] [CrossRef]
- Carroll, F.A.; Green, D.K.; Sloop, J.C. The norbornadienedicarboxylic acid-quadricyclanedicarboxylic acid water soluble solar energy storage system. Sol. Energy 1984, 33, 377–378. [Google Scholar] [CrossRef]
- Maruyama, K.; Tamiaki, H. A Water-Soluble Solar Energy Storage System. Chem. Lett. 1982, 11, 1699–1702. [Google Scholar] [CrossRef]
- Maruyama, K.; Tamiaki, H.; Yanai, T. Valence Isomerization between Water-soluble Norbornadiene and Quadricyclane derivative. Bull. Chem. Soc. Jpn. 1985, 58, 781–782. [Google Scholar] [CrossRef]
- Tamiaki, H.; Maruyama, K. A Water-Stable Quadricyclane Derivative. Chem. Lett. 1988, 17, 1875–1976. [Google Scholar] [CrossRef]
- Maruyama, K.; Tamiaki, H.; Kawabata, S. Insoluble Catalyst for Heat-Gain in a Water-Soluble Solar Energy Storage System. Chem. Lett. 1984, 13, 743–746. [Google Scholar] [CrossRef]
- Maruyama, K.; Tamiaki, H.; Kawabata, S. Development of a solar energy storage process. Photoisomerization of a norbornadiene derivative to a quadricyclane derivative in an aqueous alkaline solution. J. Org. Chem. 1985, 50, 4742–4749. [Google Scholar] [CrossRef]
- King, R.B.; Ikai, S. Metal dithiolenes as catalysts for quadricyclane reactions. J. Mol. Catal. 1978, 4, 361–373. [Google Scholar] [CrossRef]
- Behr, A.; Keim, W.; Thelen, G.; Scharf, H.-D.; Ressler, I. Solar-energy storage with quadricyclane systems. J. Chem. Tech. Biotechnol. 1982, 32, 627–630. [Google Scholar] [CrossRef]
- Cristol, S.J.; Snell, R.L. Bridged Polycyclic Compounds. VI. The Photoisomerization of Bicyclo [1,2,2]hepta-2,5-diene-2,3-dicarboxylic Acid to Quadricyclo [1,2,2,2,3,5,6]heptane-2,3-dicarboxylic Acid. J. Am. Chem. Soc. 1958, 80, 1950–1952. [Google Scholar] [CrossRef]
- Dubonosov, A.D.; Bren, V.A.; Chernoivanov, V.A. Norbornadiene−quadricyclane as an abiotic system for the storage of solar energy. Russ. Chem. Rev. 2002, 71, 917–927. [Google Scholar] [CrossRef]
- Delaude, L.; Demonceau, A.; Noels, A.F. Highly Stereoselective Ruthenium-Catalyzed Ring-Opening Metathesis Polymerization of 2,3-Difunctionalized Norbornadienes and their 7-Oxa Analogues. Macromolecules 1999, 32, 2091–2103. [Google Scholar] [CrossRef]
- Niwayama, S. Highly Efficient Selective Monohydrolysis of Symmetric Diesters. J. Org. Chem. 2000, 65, 5834–5836. [Google Scholar] [CrossRef] [PubMed]
- Colobbio, M.; Duarte, G.; Melian, M.E.; Silvera, M.; Teixeira, R.; Dominguez, L.; Ramos, J.C.; Manta, E. First Multigram Scale-Up and Synthesis of Novel Valerolactam-Benzimidazole Hybrid Anthelmintic. Lett. Drug Des. Discov. 2023, 20, 225–231. [Google Scholar]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Martínez-Camarena, Á.; Savastano, M.; Blasco, S.; Delgado-Pinar, E.; Giorgi, C.; Bianchi, B.; García-España, E.; Bazzicalupi, C. Assembly of Polyiodide Networks with Cu(II) Complexes of Pyridinol-Based Tetraaza Macrocycles. Inorg. Chem. 2022, 61, 368–383. [Google Scholar] [CrossRef]
- Hall, J.P.; Izatt, R.M.; Christensen, J.J. A Calorimetric Study of the Heat of Ionization of Water at 25°. J. Phys. Chem. 1963, 67, 2605–2608. [Google Scholar] [CrossRef]
- Krȩżel, A.; Bal, W. A formula for correlating pKa values determined in D2O and H2O. J. Inorg. Biochem. 2004, 98, 161–166. [Google Scholar] [CrossRef]
- Stranius, K.; Börjesson, K. Determining the Photoisomerization Quantum Yield of Photoswitchable Molecules in Solution and in the Solid State. Sci. Rep. 2017, 7, 41145. [Google Scholar] [CrossRef]







| HNBD1/NBD1− | |||
| pKa a | λonset a (nm) | Φiso a (%) | ΔHstorage b (kJ∙mol−1) |
| 3.73(1) | 364 (pH 2); 343 (pH 11) | 71 | 65(2) |
| HQC1/QC1− | |||
| pKa a | t½c Thermal, 298 K | t½c Thermal, 371 K | t½c Cat. Co-TPPC, 298 K |
| 3.60(1) | 100% stable over 3 months | 587 h | 11 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, F.; Gancheff, J.S.; Ramos, J.C.; Seoane, G.; Bazzicalupi, C.; Bianchi, A.; Ridi, F.; Savastano, M. A Norbornadiene-Based Molecular System for the Storage of Solar–Thermal Energy in an Aqueous Solution: Study of the Heat-Release Process Triggered by a Co(II)-Complex. Molecules 2023, 28, 7270. https://doi.org/10.3390/molecules28217270
Castro F, Gancheff JS, Ramos JC, Seoane G, Bazzicalupi C, Bianchi A, Ridi F, Savastano M. A Norbornadiene-Based Molecular System for the Storage of Solar–Thermal Energy in an Aqueous Solution: Study of the Heat-Release Process Triggered by a Co(II)-Complex. Molecules. 2023; 28(21):7270. https://doi.org/10.3390/molecules28217270
Chicago/Turabian StyleCastro, Franco, Jorge S. Gancheff, Juan C. Ramos, Gustavo Seoane, Carla Bazzicalupi, Antonio Bianchi, Francesca Ridi, and Matteo Savastano. 2023. "A Norbornadiene-Based Molecular System for the Storage of Solar–Thermal Energy in an Aqueous Solution: Study of the Heat-Release Process Triggered by a Co(II)-Complex" Molecules 28, no. 21: 7270. https://doi.org/10.3390/molecules28217270
APA StyleCastro, F., Gancheff, J. S., Ramos, J. C., Seoane, G., Bazzicalupi, C., Bianchi, A., Ridi, F., & Savastano, M. (2023). A Norbornadiene-Based Molecular System for the Storage of Solar–Thermal Energy in an Aqueous Solution: Study of the Heat-Release Process Triggered by a Co(II)-Complex. Molecules, 28(21), 7270. https://doi.org/10.3390/molecules28217270