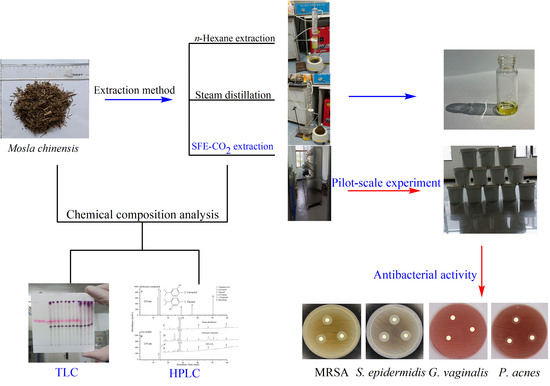
HPLC–DAD Analysis, SFE-CO2 Extraction, and Antibacterial Activity on Bioactive Compounds from Mosla chinensis Maxim
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analysis of M. chinensis by TLC and HPLC–DAD
2.2. Solid–Liquid Ratio and Extraction Time Results for Steam Distillation and n-Hexane Extraction Rate
2.3. Optimization Results of SFE-CO2 Extraction by Single Factor and RSM
2.3.1. Single-Factor Results of Temperature, Pressure, and Time for Extraction Rate
2.3.2. The RSM Results, Variance Analysis, and Verification of SFE-CO2
2.4. Antibacterial Activity of Extract Sample from SFE-CO2
3. Materials and Methods
3.1. Chemical Reagents, Apparatus, and Materials
3.2. Solid–Liquid Ratio and Time Investigation for Steam Distillation and n-Hexane Extraction
3.3. SFE-CO2 Extraction Results for Bioactive Compounds from M. chinensis
3.3.1. Single-Factor Results of Extraction Temperature, Pressure, and Time
3.3.2. Response Surface Optimization
3.4. Chemical Composition Analysis
3.5. Antibacterial Activity of Bioactive Compounds from M. chinensis
3.5.1. Microbial Strains
3.5.2. Antimicrobial Circle Screening
3.5.3. Determination of the Minimum Inhibitory Concentration (MIC)
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Wang, H.; Wang, F.; Li, H.; Cao, F.; Luo, D.; Zhang, Q.; Chen, F. Isolation of essential oil from Mosla chinensis Maxim by surfactant-enzyme pretreatment in high-solid system and evaluation of its biological activity. Ind. Crops Prod. 2022, 189, 115871. [Google Scholar] [CrossRef]
- Lu, X.; Weng, H.; Li, C.; He, J.; Zhang, X.; Ma, Z. Efficacy of essential oil from Mosla chinensis Maxim. cv. Jiangxiangru and its three main components against insect pests. Ind. Crops Prod. 2020, 147, 112237. [Google Scholar] [CrossRef]
- Cao, L.; Si, J.Y.; Liu, Y.; Sun, H.; Jin, W.; Li, Z.; Zhao, X.H.; Pan, R.L. Essential oil composition, antimicrobial and antioxidant properties of Mosla chinensis Maxim. Food Chem. 2009, 115, 801–805. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, S.; Lv, Y.; Pang, P.; Deng, L.; Xu, H.; Shi, Y.; Chen, X. Carvacrol inhibits the excessive immune response induced by influenza virus A via suppressing viral replication and TLR/RLR pattern recognition. J. Ethnopharmacol. 2021, 268, 113555. [Google Scholar] [CrossRef]
- Zhong, J.; Muhammad, N.; Yang, X.; Li, J. Antimicrobial, antioxidant, anti-inflammatory activities of eight essential oils obtained from traditional Chinese medicines using supercritical fluid extraction coupled molecular distillation. J. Essent. Oil-Bear. Plants 2022, 25, 1145–1158. [Google Scholar] [CrossRef]
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Liu, M.T.; Luo, F.Y.; Qing, Z.X.; Yang, H.C.; Liu, X.B.; Yang, Z.H.; Zeng, J.G. Chemical composition and bioactivity of essential oil of ten Labiatae species. Molecules 2020, 25, 4862. [Google Scholar] [CrossRef]
- Kim, T.H.; Thuy, N.T.; Shin, J.H.; Baek, H.H.; Lee, H.J. Aroma-active compounds of miniature beefsteakplant (Mosla dianthera Maxim.). J. Agric. Food Chem. 2000, 48, 2877–2881. [Google Scholar] [CrossRef]
- Móricz, A.M.; Häbe, T.T.; Böszörményi, A.; Ott, P.G.; Morlock, G.E. Tracking and identification of antibacterial components in the essential oil of Tanacetum vulgare L. by the combination of high-performance thin-layer chromatography with direct bioautography and mass spectrometry. J. Chromatogr. A 2015, 1422, 310–317. [Google Scholar] [CrossRef]
- Lebanov, L.; Lam, S.C.; Tadone, L.; Sostaric, T.; Smith, J.A.; Ghiasvand, A.; Paull, B. Radical scavenging activity and metabolomic profiling study of ylang-ylang essential oils based on high-performance thin-layer chromatography and multivariate statistical analysis. J. Chromatogr. B 2021, 1179, 122861. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Peng, L.; Liang, Z.; Yan, X.; Zhu, Y.; Liu, Y. Comparison of essential oil composition and phenolic acid content of selected Salvia species measured by GC-MS and HPLC methods. Ind. Crops Prod. 2015, 69, 329–334. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Guerra, L.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind. Crops Prod. 2020, 149, 112329. [Google Scholar] [CrossRef]
- Behaiyn, S.; Ebrahimi, S.N.; Rahimi, M.; Behboudi, H. Response surface methodology optimization extraction of aloins from Aloe vera leaf skin by ultrasonic horn sonicator and cytotoxicity evaluation. Ind. Crops Prod. 2023, 202, 117043. [Google Scholar] [CrossRef]
- Wang, F.; You, H.; Guo, Y.; Wei, Y.; Xia, P.; Yang, Z.; Ren, M.; Guo, H.; Han, R.; Yang, D. Essential oils from three kinds of fingered citrons and their antibacterial activities. Ind. Crops Prod. 2020, 147, 112172. [Google Scholar] [CrossRef]
- Peng, L.; Xiong, Y.; Wang, M.; Han, M.M.; Cai, W.L.; Li, Z.M. Chemical composition of essential oil in Mosla chinensis Maxim cv. Jiangxiangru and its inhibitory effect on Staphylococcus aureus biofilm formation. Open Life Sci. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Lorenzo, A.D.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- Xiang, F.; Bai, J.; Tan, X.; Chen, T.; Yang, W.; He, F. Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Ind. Crops Prod. 2018, 125, 582–587. [Google Scholar] [CrossRef]
- Silva, A.R.P.; Costa, M.C.; Araújo, N.J.S.; Freitas, T.S.; Santos, A.T.L.; Gonçalves, S.A.; Silva, V.B.; Andrade-Pinheiro, J.C.; Tahim, C.M.; Lucetti, E.C.P.; et al. Antibacterial activity and antibiotic-modifying action of carvacrol against multidrug-resistant bacteria. Adv. Sample Prep. 2023, 7, 100072. [Google Scholar] [CrossRef]
- Dutra, T.V.; Castro, J.C.; Menezes, J.L.; Ramos, T.R.; Prado, I.N.; Junior, M.M.; Mikcha, J.M.G.; Filho, B.A.A. Bioactivity of oregano (Origanum vulgare) essential oil against Alicyclobacillus spp. Ind. Crops Prod. 2019, 129, 345–349. [Google Scholar] [CrossRef]
- Sokmen, A.; Gulluce, M.; Akpulat, H.A.; Daferera, D.; Tepe, B.; Polissiou, M.; Sokmen, M.; Sahin, F. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 2004, 15, 627–634. [Google Scholar] [CrossRef]




| No. | Component | Molecular Formula | CAS Number | Retention Time (min) | Relative Content Ratio b (%) | ||
|---|---|---|---|---|---|---|---|
| Steam Distillation | n-Hexane Extraction | SFE-CO2 | |||||
| 1 | Terpinen-4-ol | C10H18O | 562-74-3 | 12.973 | 0.13 | 0.12 | 0.12 |
| 2 | Carvacrol | C10H14O | 499-75-2 | 13.87 | 12.03 | 10.62 | 8.13 |
| 3 | Thymol | C10H14O | 89-83-8 | 14.773 | 82.21 | 69.58 | 74.96 |
| 4 | p-Cymene | C10H14 | 99-87-6 | 28.994 | 1.03 | 0.2 | 1.83 |
| 5 | γ-Terpinene | C10H16 | 99-85-4 | 36.313 | 0.34 | 4.93 c | 4.27 c |
| 6 | Humulene | C15H24 | 6753-98-6 | 50.562 | 0.92 | 0.33 | 1.29 |
| Total identified components | 96.67 | 85.78 | 90.6 | ||||
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 9.6 | 9 | 1.07 | 535.18 | <0.0001 | significant |
| A—temperature | 0.00405 | 1 | 0.00405 | 2.03 | 0.1971 | |
| B—pressure | 0.88 | 1 | 0.88 | 440.32 | <0.0001 | |
| C—time | 0.92 | 1 | 0.92 | 460.49 | <0.0001 | |
| AB | 0.0004 | 1 | 0.0004 | 0.2 | 0.6677 | |
| AC | 0.0016 | 1 | 0.0016 | 0.8 | 0.4001 | |
| BC | 0.042 | 1 | 0.042 | 21.08 | 0.0025 | |
| A2 | 0.98 | 1 | 0.98 | 491.19 | <0.0001 | |
| B2 | 5.61 | 1 | 5.61 | 2816.31 | <0.0001 | |
| C2 | 0.58 | 1 | 0.58 | 288.75 | <0.0001 | |
| Residual | 0.014 | 7 | 0.00199 | |||
| Lack of fit | 0.00808 | 3 | 0.00269 | 1.83 | 0.2816 | not significant |
| Pure error | 0.00588 | 4 | 0.00147 | |||
| Cor total | 9.62 | 16 |
| Name | Antibacterial Circle Diameter (mm) b | MIC c (µg/mL) | |
|---|---|---|---|
| Tetracycline | |||
| Gardnerella vaginalis | 13.71 ± 0.49 | 16 | 2 |
| Propionibacterium acnes | 14.17 ± 0.17 | 32 | 1 |
| Staphylococcus aureus | 15.11 ± 0.78 | 16 | <1 |
| Staphylococcus epidermidis | 12.55 ± 0.53 | 32 | <1 |
| methicillin-resistant Staphylococcus aureus | 14.89 ± 0.78 | 16 | <1 |
| Aspergillus flavus | - d | ||
| Epidermophyton floccosum | - | ||
| Helicobacter pylori | - | ||
| Malassezia furfur | - | ||
| Run | Factor | |||
|---|---|---|---|---|
| Temperature (°C) | Pressure (MPa) | Time (h) | Extraction Rate (%) | |
| 1 | 50 | 10 | 1.5 | 1.23 |
| 2 | 45 | 15 | 1.5 | 3.2 |
| 3 | 40 | 20 | 1.5 | 1.98 |
| 4 | 50 | 20 | 1.5 | 1.97 |
| 5 | 45 | 20 | 1.0 | 1.6 |
| 6 | 45 | 15 | 1.5 | 3.23 |
| 7 | 50 | 15 | 1.0 | 2.04 |
| 8 | 40 | 15 | 1.0 | 2.06 |
| 9 | 40 | 15 | 2.0 | 2.8 |
| 10 | 40 | 10 | 1.5 | 1.28 |
| 11 | 45 | 15 | 1.5 | 3.3 |
| 12 | 45 | 20 | 2.0 | 2.46 |
| 13 | 45 | 15 | 1.5 | 3.26 |
| 14 | 45 | 15 | 1.5 | 3.27 |
| 15 | 45 | 10 | 2.0 | 1.65 |
| 16 | 45 | 10 | 1.0 | 1.2 |
| 17 | 50 | 15 | 2.0 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Han, B.; Zeng, Y.; Shen, L.; Liu, X.; Wang, Q.; Liao, M.; Li, J. HPLC–DAD Analysis, SFE-CO2 Extraction, and Antibacterial Activity on Bioactive Compounds from Mosla chinensis Maxim. Molecules 2023, 28, 7724. https://doi.org/10.3390/molecules28237724
Gao R, Han B, Zeng Y, Shen L, Liu X, Wang Q, Liao M, Li J. HPLC–DAD Analysis, SFE-CO2 Extraction, and Antibacterial Activity on Bioactive Compounds from Mosla chinensis Maxim. Molecules. 2023; 28(23):7724. https://doi.org/10.3390/molecules28237724
Chicago/Turabian StyleGao, Ruixi, Bingchen Han, Yanfeng Zeng, Linchuang Shen, Xinqiao Liu, Qiang Wang, Maochuan Liao, and Jun Li. 2023. "HPLC–DAD Analysis, SFE-CO2 Extraction, and Antibacterial Activity on Bioactive Compounds from Mosla chinensis Maxim" Molecules 28, no. 23: 7724. https://doi.org/10.3390/molecules28237724
APA StyleGao, R., Han, B., Zeng, Y., Shen, L., Liu, X., Wang, Q., Liao, M., & Li, J. (2023). HPLC–DAD Analysis, SFE-CO2 Extraction, and Antibacterial Activity on Bioactive Compounds from Mosla chinensis Maxim. Molecules, 28(23), 7724. https://doi.org/10.3390/molecules28237724