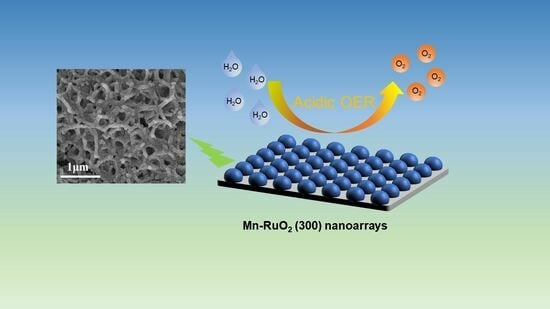
Self–Supporting Mn–RuO2 Nanoarrays for Stable Oxygen Evolution Reaction in Acid
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. Materials
3.2. Preparation of Mn–RuO2 Self–Supporting Electrode
3.3. Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Jiang, Y.; An, T.; Cao, M. Increasing the active sites and intrinsic activity of transition metal chalcogenide electrocatalysts for enhanced water splitting. J. Mater. Chem. A 2020, 8, 25465–25498. [Google Scholar] [CrossRef]
- Joshi, K.; Mistry, K.; Tripathi, B.; Chandra, P.; Shinde, S.M.; Kumar, M.; Santola, D.; Chokshi, H.; Gurrala, P. MoS2 Nanostructures for Solar Hydrogen Generation via Membraneless Electrochemical Water Splitting. ACS Appl. Electron. 2023, 5, 1461–1470. [Google Scholar] [CrossRef]
- Su, H.; Jiang, J.; Song, S.; An, B.; Li, N.; Gao, Y.; Ge, L. Recent progress on design and applications of transition metal chalcogenide-associated electrocatalysts for the overall water splitting. Chin. J. Catal. 2023, 44, 7–49. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Yang, Y.; Sirisomboonchai, S.; Wang, P.; Ma, X.; Abudula, A.; Guan, G. Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: A review. J. Alloys Compd. 2020, 819, 155346. [Google Scholar] [CrossRef]
- Su, J.; Ge, R.; Jiang, K.; Dong, Y.; Hao, F.; Tian, Z.; Chen, G.; Chen, L. Assembling Ultrasmall Copper-Doped Ruthenium Oxide Nanocrystals into Hollow Porous Polyhedra: Highly Robust Electrocatalysts for Oxygen Evolution in Acidic Media. Adv. Mater. 2018, 30, 1801351. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, B.; Su, J.; Zhao, K.; Chen, L. MOF-Derived Zinc-Doped Ruthenium Oxide Hollow Nanorods as Highly Active and Stable Electrocatalysts for Oxygen Evolution in Acidic Media. ChemNanoMat 2021, 7, 117–121. [Google Scholar] [CrossRef]
- Kiessling, A.; Fornaciari, J.C.; Anderson, G.; Peng, X.; Gerstmayr, A.; Gerhardt, M.R.; McKinney, S.; Serov, A.; Kim, Y.S.; Zulevi, B.; et al. Influence of Supporting Electrolyte on Hydroxide Exchange Membrane Water Electrolysis Performance: Anolyte. J. Electrochem. Soc. 2021, 168, 1945–7111. [Google Scholar] [CrossRef]
- Lotric, A.; Sekavcnik, M.; Kustrin, I.; Mori, M. Life-cycle assessment of hydrogen technologies with the focus on EU critical raw materials and end-of-life strategies. Int. J. Hydrogen Energy 2021, 46, 10143–10160. [Google Scholar] [CrossRef]
- Kokoh, K.B.; Mayousse, E.; Napporn, T.W.; Servat, K.; Guillet, N.; Soyez, E.; Grosjean, A.; Rakotondrainibe, A.; Paul-Joseph, J. Efficient multi-metallic anode catalysts in a PEM water electrolyzer. Int. J. Hydrogen Energy 2014, 39, 1924–1931. [Google Scholar] [CrossRef]
- Peng, Y.; Liao, Y.; Ye, D.; Meng, Z.; Wang, R.; Zhao, S.; Tian, T.; Tang, H. Recent Advances Regarding Precious Metal-Based Electrocatalysts for Acidic Water Splitting. Nanomaterials 2022, 12, 2618–2644. [Google Scholar] [CrossRef]
- Hu, E.; Yao, Y.; Chen, Y.; Cui, Y.; Wang, Z.; Qian, G. Boosting hydrogen generation by anodic oxidation of iodide over Ni-Co(OH)2 nanosheet arrays. Nanoscale Adv. 2021, 3, 604–610. [Google Scholar] [CrossRef]
- Lin, Y.; Dong, Y.; Wang, X.; Chen, L. Electrocatalysts for the Oxygen Evolution Reaction in Acidic Media. Adv. Mater. 2023, 35, 2210565. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, X.; Ge, J.; Liu, C.; Xing, W. Fundamental understanding of the acidic oxygen evolution reaction: Mechanism study and state-of-the-art catalysts. Nanoscale 2020, 12, 13249–13275. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Danilovic, N.; Wang, Y.; Willis, W.; Poozhikunnath, A.; Bonville, L.; Capuano, C.; Ayers, K.; Maric, R. Nano-size IrOx catalyst of high activity and stability in PEM water electrolyzer with ultra-low iridium loading. Appl. Catal. B 2018, 239, 133–146. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability. Catal. Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Sun, H.; Jung, W. Recent advances in doped ruthenium oxides as high-efficiency electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 15506–15521. [Google Scholar] [CrossRef]
- Exner, K.S. Boosting the Stability of RuO2 in the Acidic Oxygen Evolution Reaction by Tuning Oxygen-Vacancy Formation Energies: A Viable Approach Beyond Noble-Metal Catalysts? ChemElectroChem 2021, 8, 46–48. [Google Scholar] [CrossRef]
- Qu, M.J.; Jiang, Y.M.; Yang, M.; Liu, S.; Guo, Q.F.; Shen, W.; Li, M.; He, R.X. Regulating electron density of NiFe-P nanosheets electrocatalysts by a trifle of Ru for high-efficient overall water splitting. Appl. Catal. B 2020, 263, 118324. [Google Scholar] [CrossRef]
- He, Y.; Yan, F.; Zhang, X.; Zhu, C.; Zhao, Y.; Geng, B.; Chou, S.; Xie, Y.; Chen, Y. Creating Dual Active Sites in Conductive Metal-Organic Frameworks for Efficient Water Splitting. Adv. Energy Mater. 2023, 13, 202204177. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chen, H.; Li, G.; Peng, J.H.; Zhang, Y.X. One-pot synthesis of pearl-chain-like manganese dioxide-decorated titanium grids as advanced binder-free supercapacitors electrodes. Ceram. Int. 2016, 42, 9227–9233. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, Y.; Gu, Y.; Lian, Y.; Su, Y.; Hu, J.; Zhao, X.; Peng, Y.; Feng, K.; et al. Ru-Substituted MnO2 for Accelerated Water Oxidation: The Feedback of Strain-Induced and Polymorph-Dependent Structural Changes to the Catalytic Activity and Mechanism. ACS Catal. 2023, 13, 256–266. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Han, A.; Wang, J.; Tan, H.-R.; Tok, E.-S.; Jaenicke, S.; Chuah, G.-K. Ru/ZrO2 Catalysts for Transfer Hydrogenation of Levulinic Acid with Formic Acid/Formate Mixtures: Importance of Support Stability. ChemistrySelect 2018, 3, 1343–1351. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, X.; Liu, M.; Liu, J.; Geng, Z.; Cao, R.; Zhang, X.; Zhang, W.; Huang, K.; Feng, S. Silver-Intermediated Perovskite La0.9FeO3-δtoward High-Performance Cathode Catalysts for Nonaqueous Lithium-Oxygen Batteries. ACS Catal. 2019, 9, 11743–11752. [Google Scholar] [CrossRef]
- Qiu, L.; Zheng, G.; He, Y.; Lei, L.; Zhang, X. Ultra-small Sn-RuO2 nanoparticles supported on N-doped carbon polyhedra for highly active and durable oxygen evolution reaction in acidic media. Chem. Eng. J. 2021, 409, 126155. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, D.; Zhai, T.; Liu, Z.; Huang, Y.; Xie, S.; Tong, Y. Facile synthesis of large-area manganese oxide nanorod arrays as a high-performance electrochemical supercapacitor. Energy Environ. Sci. 2011, 4, 2915–2921. [Google Scholar] [CrossRef]
- Audichon, T.; Guenot, B.; Baranton, S.; Cretin, M.; Lamy, C.; Coutanceau, C. Effect of the annealing atmosphere on the electrochemical properties of RuO2 nano-oxides synthesized by the Instant Method. Appl. Catal. B 2017, 218, 385–397. [Google Scholar] [CrossRef]
- Morgen, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Lin, C.; Li, J.-L.; Li, X.; Yang, S.; Luo, W.; Zhang, Y.; Kim, S.-H.; Kim, D.-H.; Shinde, S.S.; Li, Y.F.; et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 2021, 4, 1012–1023. [Google Scholar] [CrossRef]
- Cai, S.; Zheng, M.; Lin, X.; Lei, M.; Yuan, R.; Dong, Q. A Synergistic Catalytic Mechanism for Oxygen Evolution Reaction in Aprotic Li-O2 Battery. ACS Catal. 2018, 8, 7983–7990. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, S.H.; Kim, S.H.; Ko, Y.; Kang, K.; Lee, Y.J. High-Rate and High-Areal-Capacity Air Cathodes with Enhanced Cycle Life Based on RuO2/MnO2 Bifunctional Electrocatalysts Supported on CNT for Pragmatic Li-O2 Batteries. ACS Catal. 2018, 8, 2923–2934. [Google Scholar] [CrossRef]
- Jin, H.Y.; Liu, X.Y.; An, P.F.; Tang, C.; Yu, H.M.; Zhang, Q.H.; Peng, H.J.; Gu, L.; Zheng, Y.; Song, T.S.; et al. Dynamic rhenium dopant boosts ruthenium oxide for durable oxygen evolution. Nat. Commun. 2023, 14, 354–365. [Google Scholar] [CrossRef]
- Geiger, S.; Kasian, O.; Ledendecker, M.; Pizzutilo, E.; Mingers, A.M.; Fu, W.T.; Diaz-Morales, O.; Li, Z.; Oellers, T.; Fruchter, L.; et al. The stability number as a metric for electrocatalyst stability benchmarking. Nat. Catal. 2018, 1, 508–515. [Google Scholar] [CrossRef]
- Zhang, D.; Li, M.; Yong, X.; Song, H.; Waterhouse, G.I.N.; Yi, Y.; Xue, B.; Zhang, D.; Liu, B.; Lu, S. Construction of Zn-doped RuO2 nanowires for efficient and stable water oxidation in acidic media. Nat. Commun. 2023, 14, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Hung, S.-F.; Lin, Z.Y.; Zhang, Y.; Zhang, C.; Hao, Y.; Liu, S.; Kuo, C.H.; Chen, H.Y.; Peng, J.; et al. Valence Oscillation of Ru Active Sites for Efficient and Robust Acidic Water Oxidation. Adv. Mater. 2023, e2305939. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Ding, Y.; Zhang, B.; Li, H.; Bai, B.; Li, M.; Cui, Y.; Xiao, J.; Wu, Z.-S. Unraveling oxygen vacancy site mechanism of Rh-doped RuO2 catalyst for long-lasting acidic water oxidation. Nat. Commun. 2023, 14, 1412–1422. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Zhang, B.; Fan, D.Y.; Wang, Y.G.; Yang, H.J.; Huang, K.; Pan, X.C.; Zhang, R.M.; Tang, H.L.; Lei, M. Tailoring manganese oxide nanoplates enhances oxygen evolution catalysis in acid. J. Catal. 2022, 406, 265–272. [Google Scholar] [CrossRef]
- Cang, B.; Guo, Y.; Kim, J.; Whitten, A.E.; Wood, K.; Kani, K.; Rowan, A.E.; Henzie, J.; Yamauchi, Y. Mesoporous Metallic Iridium Nanosheets. J. Am. Chem. Soc. 2018, 140, 12434–12441. [Google Scholar]
- Chen, S.; Huang, H.; Jiang, P.; Yang, K.; Diao, J.; Gong, S.; Liu, S.; Huang, M.; Wang, H.; Chen, Q. Mn-Doped RuO2 Nanocrystals as Highly Active Electrocatalysts for Enhanced Oxygen Evolution in Acidic Media. ACS Catal. 2020, 10, 1152–1160. [Google Scholar] [CrossRef]
- Luo, F.; Hu, H.; Zhao, X.; Yang, Z.; Zhang, Q.; Xu, J.; Kaneko, T.; Yoshida, Y.; Zhu, C.; Cai, W. Robust and Stable Acidic Overall Water Splitting on Ir Single Atoms. Nano Lett. Nano Let. 2020, 20, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.L.; Luo, Q.Q.; Chen, J.J.; Wang, L.; Lin, Y.; Wang, H.J.; Liu, X.K.; Shen, X.Y.; Zhang, W.; Liu, W.; et al. Dynamic oxygen adsorption on single-atomic Ruthenium catalyst with high performance for acidic oxygen evolution reaction. Nat. Commun. 2019, 10, 4849–4858. [Google Scholar] [CrossRef]
- Shan, J.; Ye, C.; Chen, S.; Sun, T.; Jiao, Y.; Liu, L.; Zhu, C.; Song, L.; Han, Y.; Jaroniec, M.; et al. Short-Range ordered iridium single atoms integrated into cobalt oxide spinel structure for highly efficient electrocatalytic water oxidation. J. Am. Chem. Soc. 2021, 143, 5201–5211. [Google Scholar] [CrossRef]
- Shi, C.X.; Yuan, Y.; Shen, Q.; Yang, X.D.; Cao, B.Q.; Xu, B.; Kang, B.T.; Sun, Y.Q.; Li, C.C. Encapsulated ruthenium nanoparticles activated few-layer carbon frameworks as high robust oxygen evolution electrocatalysts in acidic media. J. Colloid Interface Sci. 2022, 612, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; He, F.X.; Shao, R.Y.; Yan, Q.Q.; Yin, P.; Zeng, W.J.; Zuo, M.; He, L.X.; Liang, H.W. Intermetallic IrGa-IrOx core-shell electrocatalysts for oxygen evolution. Nano Res. 2022, 15, 1853–1860. [Google Scholar] [CrossRef]
- Xu, J.; Lian, Z.; Wei, B.; Li, Y.; Bondarchuk, O.; Zhang, N.; Yu, Z.; Araujo, A.; Amorim, I.; Wang, Z.; et al. Strong Electronic Coupling between Ultrafine Iridium-Ruthenium Nanoclusters and Conductive, Acid-Stable Tellurium Nanoparticle Support for Efficient and Durable Oxygen Evolution in Acidic and Neutral Media. ACS Catal. 2020, 10, 3571–3579. [Google Scholar] [CrossRef]
- Niu, S.Q.; Kong, X.P.; Li, S.W.; Zhang, Y.Y.; Wu, J.; Zhao, W.W.; Xu, P. Low Ru loading RuO2/(Co,Mn)(3)Ox-4 nanocomposite with modulated electronic structure for efficient oxygen evolution reaction in acid. Appl. Catal. B 2021, 297, 926–934. [Google Scholar] [CrossRef]
- Qin, Y.; Cao, B.; Zhou, X.-Y.; Xiao, Z.; Zhou, H.; Zhao, Z.; Weng, Y.; Lv, J.; Liu, Y.; He, Y.-B.; et al. Orthorhombic (Ru, Mn)2O3: A superior electrocatalyst for acidic oxygen evolution reaction. Nano Energy 2023, 115, 108727. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, M.; Tang, Y.; Lu, Z.; Wang, Y.; Lin, Y. Self–Supporting Mn–RuO2 Nanoarrays for Stable Oxygen Evolution Reaction in Acid. Molecules 2023, 28, 7727. https://doi.org/10.3390/molecules28237727
Deng M, Tang Y, Lu Z, Wang Y, Lin Y. Self–Supporting Mn–RuO2 Nanoarrays for Stable Oxygen Evolution Reaction in Acid. Molecules. 2023; 28(23):7727. https://doi.org/10.3390/molecules28237727
Chicago/Turabian StyleDeng, Mengting, Yulong Tang, Zhiyi Lu, Yunan Wang, and Yichao Lin. 2023. "Self–Supporting Mn–RuO2 Nanoarrays for Stable Oxygen Evolution Reaction in Acid" Molecules 28, no. 23: 7727. https://doi.org/10.3390/molecules28237727
APA StyleDeng, M., Tang, Y., Lu, Z., Wang, Y., & Lin, Y. (2023). Self–Supporting Mn–RuO2 Nanoarrays for Stable Oxygen Evolution Reaction in Acid. Molecules, 28(23), 7727. https://doi.org/10.3390/molecules28237727