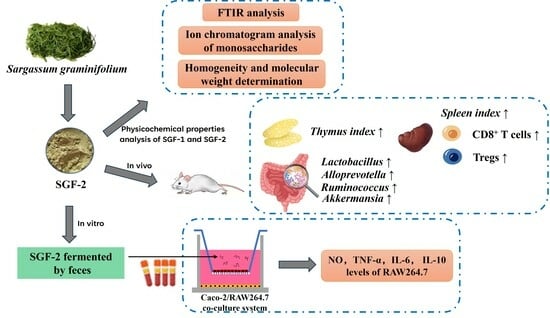
Study on Immunoregulatory Effects of Fucoidan from Sargassum graminifolium In Vivo and Immunoactivation Activity of Its Fecal Fermentation Products Using Co-Culture Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification and Characterization of SGF−1 and SGF−2
2.2. The Effects of SGF−2 on Immune Organ Indices
2.3. Modulation of Gut Microbiota by SGF−2
2.4. Regulatory Effect of SGF−2 on Immune Cell Subpopulations
2.5. Effect of SGF−2 with Fecal Fermentation on the Immunoregulatory Activity in a Co-Culture Model
2.5.1. Effects of SGF−2 with Fecal Fermentation on Cell Viability in RAW264.7 Cells
2.5.2. Effects of SGF−2 with Fecal Fermentation on Phagocytic Activity
2.5.3. Effects of SGF−2 with Fecal Fermentation on the Levels of NO and Cytokines
3. Materials and Methods
3.1. Reagents and Materials
3.2. Isolation of SGF−1 and SGF−2 from Sargassum graminifolium
3.3. Physicochemical Properties Analysis of SGF−1 and SGF−2
3.4. Animals and Experimental Design
3.5. Subpopulation Analysis of Splenocytes
3.6. Detection of Gut Microbiota
3.7. SGF−2 Fermentation In Vitro
3.8. Cell Culture
3.9. Caco-2/RAW264.7 Co-Culture Model
3.10. Cell Viability Measurements by CCK-8
3.11. NO and Cytokine Production Assay
3.12. Phagocytic Activity Assay
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Tan, H.; Nie, S. Beneficial effects of seaweed-derived dietary fiber: Highlights of the sulfated polysaccharides. Food Chem. 2022, 373, 131608. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Tagliapietra, B.L.; Clerici, M.T.P.S. Brown algae and their multiple applications as functional ingredient in food production. Food Res. Int. 2023, 167, 112655. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Kong, T.; Wu, W.-H.; Lan, M.-B. The Protection of Polysaccharide from the Brown Seaweed Sargassum graminifolium against Ethylene Glycol-Induced Mitochondrial Damage. Mar. Drugs 2013, 11, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Wu, W.H.; Wang, J.; Lan, M.B. Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Mar Drugs 2012, 10, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zeng, Q.; Zhang, Y.; Yin, Q.; Zhu, X.; Zhang, P.; Wang, C.; Liu, J. Effects of fucoidans and alginates from Sargassum graminifolium on allergic symptoms and intestinal microbiota in mice with OVA-induced food allergy. Food Funct. 2022, 12, 6702–6715. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Cheng, Y.; Guo, Y.; Hu, B.; Yao, W.; Zhu, X.; Qian, H. Study on fecal fermentation characteristics of aloe polysaccharides in vitro and their predictive modeling. Carbohydr. Polym. 2021, 256, 117571. [Google Scholar] [CrossRef]
- Luo, X.; Huang, Q.; Fu, X.; Kraithong, S.; Hu, Y.; Yuan, Y.; Bao, J.; Zhang, B. In vitro fecal fermentation characteristics of mutant rice starch depend more on amylose content than crystalline structure. Carbohydr. Polym. 2023, 307, 120606. [Google Scholar] [CrossRef]
- Bogdanov, I.V.; Finkina, E.I.; Melnikova, D.N.; Ziganshin, R.H.; Ovchinnikova, T.V. Investigation of Sensitization Potential of the Soybean Allergen Gly m 4 by Using Caco-2/Immune Cells Co-Culture Model. Nutrients 2021, 13, 2058. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; He, B.; Tan, J.; Hao, H.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, E.; Haskey, N.; Campbell, K. Chapter 4—Gut Microbiota in Health and Disease. In Gut Microbiota; Ishiguro, E., Haskey, N., Campbell, K., Eds.; Academic Press: London, UK, 2018; pp. 57–90. [Google Scholar] [CrossRef]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Turnbaugh, P.J.; Fulton, R.S.; Wollam, A.; Shah, N.; Wang, C.; Magrini, V.; Wilson, R.K.; et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6. [Google Scholar] [CrossRef]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside--from diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiao, L.-L.; Zhang, X.; Tian, W.-M.; Chen, S.; Zhang, L.-P. Anti-tumor and immunomodulating activities of proteoglycans from mycelium of Phellinus nigricans and culture medium. Int. Immunopharmacol. 2008, 8, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-O.; Zhang, W.; Du, J.-Y.; Wong, K.-W.; Oda, T.; Jiyu, G. Fucoidan Can Function as an Adjuvant In Vivo to Enhance Dendritic Cell Maturation and Function and Promote Antigen-Specific T Cell Immune Responses. PLoS ONE 2014, 9, e99396. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef]
- Green, S.J.; Mellouk, S.; Hoffman, S.L.; Meltzer, M.S.; Nacy, C.A. Cellular mechanisms of nonspecific immunity to intracellular infection: Cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol. Lett. 1990, 25, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Dabaghian, E.H.; You, S.; Yelithao, K.; Cao, R.; Rezaei, M.; Alboofetileh, M.; Bita, S. The activation of NF-κB and MAPKs signaling pathways of RAW264.7 murine macrophages and natural killer cells by fucoidan from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2020, 148, 56–67. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Lin, H.-T.; Lin, H.-Y.; Lo, S.-K. Dietary Supplementation with Low-Molecular-Weight Fucoidan Enhances Innate and Adaptive Immune Responses and Protects against Mycoplasma pneumoniae Antigen Stimulation. Mar. Drugs 2019, 17, 175. [Google Scholar] [CrossRef]
- Mao, Y.-H.; Song, A.-X.; Li, L.-Q.; Yang, Y.; Yao, Z.-P.; Wu, J.-Y. A high-molecular weight exopolysaccharide from the Cs-HK1 fungus: Ultrasonic degradation, characterization and in vitro fecal fermentation. Carbohydr. Polym. 2020, 246, 116636. [Google Scholar] [CrossRef]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Noval Rivas, M. Regulatory T cells in allergic diseases. J. Allergy Clin. Immunol. 2016, 138, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Borazjani, N.; Tabarsa, M.; You, S.; Rezaei, M. Purification, molecular properties, structural characterization, and immunomodulatory activities of water soluble polysaccharides from Sargassum angustifolium. Int. J. Biol. Macromol. 2018, 109, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, S.; Shi, W.; Qi, X.; Song, W.; Mou, J.; Yang, J. Structure characterization, antioxidant and immunoregulatory properties of a novel fucoidan from the sea cucumber Stichopus chloronotus. Carbohydr Polym 2020, 231, 115767. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Huang, Q.; Wong, K.; Yang, H. Structure, molecular conformation, and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis sclerotia. Int. J. Biol. Macromol. 2017, 94, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Díez-Municio, M.; Kolida, S.; Herrero, M.; Rastall, R.A.; Moreno, F.J. In vitro faecal fermentation of novel oligosaccharides enzymatically synthesized using microbial transglycosidases acting on sucrose. J. Funct. Foods 2016, 20, 532–544. [Google Scholar] [CrossRef]
- Liu, Q.M.; Xu, S.S.; Li, L.; Pan, T.M.; Shi, C.L.; Liu, H.; Cao, M.J.; Su, W.J.; Liu, G.M. In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis. Carbohydr. Polym. 2017, 165, 189–196. [Google Scholar] [CrossRef]







| Sample | Sugar Content (%) | Proteins (%) | Sulfate Content (%) | Mw/kDa | Monosaccharide Composition (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fuc | Gal | Glc | Xyl | Man | Glc-UA | |||||
| SGF−1 | 74.72 ± 0.37 | 0.92 ± 0.47 | 5.00 ± 0.15 | 112.96 | 40.54 | 10.50 | 2.30 | 10.93 | 22.80 | 12.93 |
| SGF−2 | 55.79 ± 0.26 | 0.69 ± 0.23 | 31.81 ± 0.99 | 258.07 | 35.57 | 21.52 | 0.28 | 7.21 | 21.77 | 13.65 |
| Group | Initial Weight (g) | Final Weight (g) | Weight Gain Percentage (%) | Spleen Index (mg/g) | Thymus Index (mg/g) |
|---|---|---|---|---|---|
| Con | 26.80 ± 0.79 | 34.82 ± 0.68 | 29.93% | 4.30 ± 0.98 | 1.63 ± 0.62 |
| FL | 26.72 ± 0.93 | 34.93 ± 0.63 | 30.72% | 4.67 ± 0.39 | 1.68 ± 0.59 |
| FH | 27.13 ± 0.56 | 35.24 ± 0.55 | 29.90% | 5.34 ± 0.58 (*) | 2.07 ± 0.75 (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Huang, L.; Huang, Y.; Tian, X.; Liu, J. Study on Immunoregulatory Effects of Fucoidan from Sargassum graminifolium In Vivo and Immunoactivation Activity of Its Fecal Fermentation Products Using Co-Culture Model. Molecules 2023, 28, 7794. https://doi.org/10.3390/molecules28237794
Wang C, Huang L, Huang Y, Tian X, Liu J. Study on Immunoregulatory Effects of Fucoidan from Sargassum graminifolium In Vivo and Immunoactivation Activity of Its Fecal Fermentation Products Using Co-Culture Model. Molecules. 2023; 28(23):7794. https://doi.org/10.3390/molecules28237794
Chicago/Turabian StyleWang, Cuifang, Lan Huang, Yaolong Huang, Xin Tian, and Jieqing Liu. 2023. "Study on Immunoregulatory Effects of Fucoidan from Sargassum graminifolium In Vivo and Immunoactivation Activity of Its Fecal Fermentation Products Using Co-Culture Model" Molecules 28, no. 23: 7794. https://doi.org/10.3390/molecules28237794
APA StyleWang, C., Huang, L., Huang, Y., Tian, X., & Liu, J. (2023). Study on Immunoregulatory Effects of Fucoidan from Sargassum graminifolium In Vivo and Immunoactivation Activity of Its Fecal Fermentation Products Using Co-Culture Model. Molecules, 28(23), 7794. https://doi.org/10.3390/molecules28237794