In Vitro Anti-Inflammatory Activity of Methyl Derivatives of Flavanone
Abstract
:1. Introduction
2. Results
2.1. The Cytotoxic Effect of 2′-methylflavanone (5B), 3′-methylflavanone (6B), 4′-methylflavanone (7B), and 6-methylflavanone (8B) on Activated RAW264.7 Macrophages
2.2. The Effect of the Flavanone, 2′-methylflavanone (5B), 3′-methylflavanone (6B), 4′-methylflavanone (7B), and 6-methylflavanone (8B) on Nitric Oxide (NO) Production in LPS-Stimulated RAW264.7 Macrophages
2.3. The Effect of the Flavanone, 2′-methylflavanone (5B), 3′-methylflavanone (6B), 4′-methylflavanone (7B), and 6-methylflavanone (8B) on the Chemiluminescence of Activated RAW264.7 Macrophages
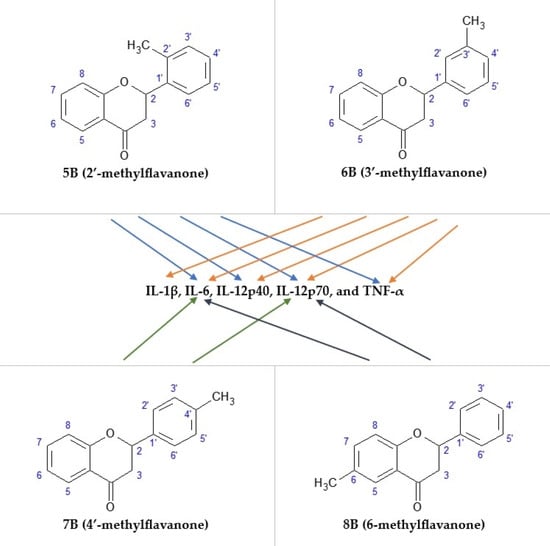
2.4. The Effect of the Flavanone, 2′-methylflavanone (5B), 3′-methylflavanone (6B), 4′-methylflavanone (7B), and 6-methylflavanone (8B) on the Release of IL-1β via Macrophages
2.5. The Effect of the Flavanone, 2′-methylflavanone, 3′-methylflavanone, 4′-methylflavanone, and 6-methylflavanone on the Release of IL-6 via Macrophages
2.6. The Effect of the Flavanone, 2′-methylflavanone, 3′-methylflavanone, 4′-methylflavanone, and 6-metyloflavanone on the Release of IL-12p40 Macrophages
2.7. The Effect of the Flavanone, 2′-methylflavanone (5B), 3′-methylflavanone (6B), 4′-methylflavanone (7B), and 6-methylflavanone (8B) on the Release of IL-12p70 Macrophages
2.8. The Effect of the Flavanone, 2′-methylflavanone, 3′-methylflavanone, 4′-methylflavanone, and 6-methylflavanone on the Release of TNF-α Macrophages
3. Discussion
4. Materials and Methods
4.1. General Procedure for the Synthesis of Methylflavanones
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Nitric Oxide Assay
4.5. Detection of ROS via Chemiluminescence
4.6. Quantification of the IL-1β, IL-6, IL-12p40, IL-12p70, and TNF-α Concentrations
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishiumi, S.; Miyamoto, S.; Kawabata, K.; Ohnishi, K.; Mukai, R.; Murakami, A.; Ashida, H.; Terao, J. Dietary flavonoids as cancer-preventive and therapeutic biofactors. Front. Biosci. 2011, 3, 1332–1362. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant. Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Molecules Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Hosseinzade, A.; Sadeghi, O.; Biregani, A.N.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory Effects of Flavonoids: Possible Induction of T CD4+ Regulatory Cells Through Suppression of mTOR Pathway Signaling Activity. Front. Immunol. 2019, 1, 51. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids regulate tumor-associated macrophages—From structure-activity relationship to clinical potential (Review). Pharmacol. Res. 2022, 184, 106419. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Zulu, A.I.; Oderinlo, O.O.; Kruger, C.; Isaacs, M.; Hoppe, H.C.; Smith, V.J.; Veale, C.G.L.; Khanye, S.D. Synthesis, structure and in vitro anti-trypanosomal activity of non-toxic arylpyrrole-based chalcone derivatives. Molecules 2020, 25, 1668. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Zill-E-Huma; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S.; Giovinazzo, G.; Gerardi, C.; Mosca, L. Molecules Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Jia, Z.; Zheng, X.; Cui, S.; Chen, W. A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends Food Sci. Technol. 2018, 79, 35–54. [Google Scholar] [CrossRef]
- Stankov, S.V. Definition of Inflammation, Causes of Inflammation and Possible Anti-inflammatory Strategies. Open Inflamm. J. 2012, 5, 1–9. [Google Scholar] [CrossRef]
- Rahaman, S.T.; Mondal, S. Flavonoids: A vital resource in healthcare and medicine. Pharm. Pharmacol. Int. J. 2020, 8, 91–104. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and ActiveBiological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Musumeci, L.; De Sarro, G.; Cirmi, S.; Navarra, M. Inflammation andObesity: The Pharmacological Role ofFlavonoids in the Zebrafish Model. Int. J. Mol. Sci. 2023, 24, 2899. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Afzal, M.; Nadeem, M.S.; Altayb, H.N. Sterubin protects against chemically-induced Alzheimer’s disease by reducing biomarkers of inflammation- IL-6/ IL-β/ TNF-α and oxidative stress- SOD/MDA in rats. Saudi J. Biol. Sci. 2023, 30, 103560. [Google Scholar] [CrossRef] [PubMed]
- Na Takuathung, M.; Potikanond, S.; Sookkhee, S.; Mungkornasawakul, P.; Jearanaikulvanich, T.; Chinda, K.; Wikan, N.; Nimlamool, W. Anti-psoriatic and anti-inflammatory effects of Kaempferia parviflora in keratinocytes and macrophage cells. Biomed. Pharmacother. 2021, 143, 112229. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, G. Naturally Occurring Flavanones: An Overview. Nat. Prod. Commun. 2008, 3, 1934578X0800300820. [Google Scholar] [CrossRef]
- Bin Emran, T.; Islam, F.; Nath, N.; Sutradhar, H.; Das, R.; Mitra, S.; Alshahrani, M.M.; Alhasaniah, A.H.; Sharma, R. Naringin and Naringenin Polyphenols in Neurological Diseases: Understandings from a Therapeutic Viewpoint. Life 2022, 13, 99. [Google Scholar] [CrossRef]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Entomopathogenic Filamentous Fungi as Biocatalysts in Glycosylation of Methylflavonoids. Catalysts 2020, 10, 1148. [Google Scholar] [CrossRef]
- Krawczyk-łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. New glycosylated dihydrochalcones obtained by biotransformation of 2′-hydroxy-2-methylchalcone in cultures of entomopathogenic filamentous fungi. Int. J. Mol. Sci. 2021, 22, 9619. [Google Scholar] [CrossRef]
- Krawczyk-Łebek, A.; Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Fungal biotransformation of 2′-methylflavanone and 2′-methylflavone as a method to obtain glycosylated derivatives. Int. J. Mol. Sci. 2021, 22, 9617. [Google Scholar] [CrossRef]
- Włoch, A.; Strugała-Danak, P.; Pruchnik, H.; Krawczyk-Łebek, A.; Szczecka, K.; Janeczko, T.; Kostrzewa-Susłow, E. Interaction of 4′-methylflavonoids with biological membranes, liposomes, and human albumin. Sci. Rep. 2021, 11, 16003. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Cancers Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of il-1 in cancer: From tumor progression to resistance to targeted therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12: A Proinflammatory Cytokine with Immunoregulatory Functions that Bridge Innate Resistance and Antigen-Specific Adaptive Immunity. Annu. Rev. Immunol. 1995, 13, 251–276. [Google Scholar] [CrossRef]
- Ichikawa, D.; Matsui, A.; Imai, M.; Sonoda, Y.; Kasahara, T. Effect of various catechins on the IL-12p40 production by murine peritoneal macrophages and a macrophage cell line, J774.1. Biol. Pharm. Bull. 2004, 27, 1353–1358. [Google Scholar] [CrossRef]
- Sakata, K.; Hirose, Y.; Qiao, Z.; Tanaka, T.; Mori, H. Inhibition of inducible isoforms of cyclooxygenase and nitric oxide synthase by flavonoid hesperidin in mouse macrophage cell line. Cancer Lett. 2003, 199, 139–145. [Google Scholar] [CrossRef]
- Wang, X.-H.; Dai, C.; Wang, J.; Liu, R.; Li, L.; Yin, Z.-S. Therapeutic effect of neohesperidin on TNF-α-stimulated human rheumatoid arthritis fibroblast-like synoviocytes. Chin. J. Nat. Med. 2021, 19, 741–749. [Google Scholar] [CrossRef]
- Shi, Q.; Song, X.; Fu, J.; Su, C.; Xia, X.; Song, E.; Song, Y. Artificial sweetener neohesperidin dihydrochalcone showed antioxidative, anti-inflammatory and anti-apoptosis effects against paraquat-induced liver injury in mice. Int. Immunopharmacol. 2015, 29, 722–729. [Google Scholar] [CrossRef]
- Lina, B.A.R.; der Meulen, H.C.D.-V.; Leegwater, D.C. Subchronic (13-week) oral toxicity of neohesperidin dihydrochalcone in rats. Food Chem. Toxicol. 1990, 28, 507–513. [Google Scholar] [CrossRef]
- Yoshida, H.; Takamura, N.; Shuto, T.; Ogata, K.; Tokunaga, J.; Kawai, K.; Kai, H. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-a in mouse adipocytes. Biochem. Biophys. Res. Commun. 2010, 394, 728–732. [Google Scholar] [CrossRef]
- Kanno, S.-I.; Shouji, A.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Ujibe, M.; Obara, Y.; Nakahata, N.; Ishikawa, M. Inhibitory effect of naringin on lipopolysaccharide (LPS)-induced endotoxin shock in mice and nitric oxide production in RAW 264.7 macrophages. Life Sci. 2006, 78, 673–681. [Google Scholar] [CrossRef]
- Gopinath, K.; Sudhandiran, G. Naringin Modulates Oxidative Stress and Inflammation In 3-Nitropropionic Acid-Induced Neurodegeneration through the Activation of Nuclear Factor-Erythroid 2-Related Factor-2 Signalling Pathway. Neuroscience 2012, 227, 134–143. [Google Scholar] [CrossRef]
- Cheng, L.; Ren, Y.; Lin, D.; Peng, S.; Zhong, B.; Ma, Z. The Anti-Inflammatory Properties of Citrus wilsonii Tanaka Extract in LPS-Induced RAW 264.7 and Primary Mouse Bone Marrow-Derived Dendritic Cells. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 1213. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Luo, X.; Yang, Z.; Drasar, P.B.; Khripach, V.A. Advances in Biosynthesis, Pharmacology, and Pharmacokinetics of Pinocembrin, a Promising Natural Small-Molecule Drug. Molecules 2019, 24, 2323. [Google Scholar] [CrossRef]
- Soromou, L.W.; Chu, X.; Jiang, L.; Wei, M.; Huo, M.; Chen, N.; Guan, S.; Yang, X.; Chen, C.; Feng, H.; et al. In vitro and in vivo protection provided by pinocembrin against lipopolysaccharide-induced inflammatory responses. Int. Immunopharmacol. 2012, 14, 66–74. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Kang, H. Sakuranetin Inhibits Inflammatory Enzyme, Cytokine, and Costimulatory Molecule Expression in Macrophages through Modulation of JNK, p38, and STAT1. Evidence-Based Complement. Altern. Med. 2016, 2016, 9824203. [Google Scholar] [CrossRef]
- Cherian, J.; Sehgal, A.; Singh, S.K.; Vamanu, E.; Singh, M.P. 2′-Hydroxyflavanone: A Bioactive Compound That Protects against Cancers. Appl. Sci. 2022, 12, 9543. [Google Scholar] [CrossRef]
- Sonowal, H.; Ramana, K.V. 2′-Hydroxyflavanone prevents LPS-induced inflammatory response and cytotoxicity in murine macrophages. Toxicol. In Vitro 2020, 69, 104966. [Google Scholar] [CrossRef]
- Kłósek, M.; Jaworska, D.; Pietsz, G.; Szliszka, E. Santin (5,7-Dihydroxy-3,6,4-Trimetoxy-Flavone) Enhances TRAIL-Mediated Apoptosis in Colon Cancer Cells. Life 2023, 13, 592. [Google Scholar] [CrossRef]
- Szliszka, E.; Skaba, D.; Czuba, Z.P.; Krol, W. Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264.7 macrophages. Molecules 2011, 16, 3701–3712. [Google Scholar] [CrossRef]
- Bronikowska, J.; Kłósek, M.; Janeczko, T.; Kostrzewa-Susłow, E.; Czuba, Z.P. The modulating effect of methoxy-derivatives of 2′-hydroxychalcones on the release of IL-8, MIF, VCAM-1 and ICAM-1 by colon cancer cells. Biomed. Pharmacother. 2022, 145, 112428. [Google Scholar] [CrossRef] [PubMed]
- Kłósek, M.; Sędek, L.; Lewandowska, H.; Czuba, Z.P. The effect of ethanolic extract of Brazilian green propolis and artepillin C on aFGF-1, Eselectin, and CD40L secreted by human gingival fibroblasts. Cent. J. Immunol. 2021, 46, 438. [Google Scholar] [CrossRef]









| Sample | IL-1β | IL-6 | IL-12p40 | IL-12p70 | TNF-α | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CV | p | CV | p | CV | p | CV | p | CV | p | |
| Control | 10.704364 | 5.478535 | 13.342202 | 5.166537 | 14.357457 | |||||
| 5B 1 μM | 8.070487 | 0.880882 | 2.756384 | 0.014881 | 16.519107 | 0.001031 | 7.895512 | 0.003850 | 11.322122 | 0.000296 |
| 5B 20 μM | 4.570054 | 0.318805 | 10.405476 | 0.000133 | 12.298108 | 0.000307 | 6.868333 | 0.000410 | 1.315403 | 0.000095 |
| 6B 1 μM | 11.353243 | 0.009652 | 6.321492 | 0.018428 | 9.332954 | 0.038380 | 5.639210 | 0.000050 | 6.345997 | 0.826990 |
| 6B 20 μM | 2.860606 | 0.006865 | 4.989167 | 0.000066 | 14.294118 | 0.010886 | 12.327883 | 0.000010 | 4.260733 | 0.974889 |
| 7B 1 μM | 21.495385 | 0.757877 | 8.755781 | 0.002296 | 6.472807 | 0.065044 | 12.839975 | 0.264156 | 9.463668 | 0.927038 |
| 7B 20 μM | 4.570054 | 0.963008 | 14.867259 | 0.804608 | 11.607130 | 0.232104 | 2.927687 | 0.005071 | 8.586474 | 0.373381 |
| 8B 1 μM | 3.760656 | 0.032619 | 20.741240 | 0.001515 | 16.148254 | 0.362711 | 8.665186 | 0.121969 | 7.928790 | 0.531949 |
| 8B 20 μM | 15.244568 | 0.612231 | 7.973248 | 0.735847 | 19.096473 | 0.365265 | 10.445151 | 0.000408 | 6.080636 | 0.069246 |
| Flavanone 1 μM | 4.485925 | 0.875184 | 21.132576 | 0.253626 | 36.649072 | 0.205639 | 7.560869 | 0.111074 | 40.729791 | 0.571884 |
| Flavanone 20 μM | 7.669079 | 0.538352 | 11.567501 | 0.005065 | 13.151849 | 0.946897 | 4.687945 | 0.570728 | 18.376227 | 0.008844 |
| Sample | IL-1β | IL-6 | IL-12p40 | IL-12p70 | TNF-α | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CV | p | CV | p | CV | p | CV | p | CV | p | |
| Flavanone 1 μM | 4.485925 | 21.132576 | 36.649072 | 7.560869 | 40.729791 | |||||
| Control | 10.704364 | 0.875184 | 5.478535 | 0.253626 | 13.342202 | 0.205639 | 5.166537 | 0.111074 | 14.357457 | 0.571884 |
| 5B 1 μM | 8.070487 | 0.994230 | 2.756384 | 0.000947 | 16.519107 | 0.021488 | 7.895512 | 0.000069 | 11.322122 | 0.000073 |
| 6B 1 μM | 11.353243 | 0.006699 | 6.321492 | 0.001197 | 9.332954 | 0.378589 | 5.639210 | 0.000001 | 6.345997 | 0.727688 |
| 7B 1 μM | 21.495385 | 0.642240 | 8.755781 | 0.032999 | 6.472807 | 0.530175 | 12.839975 | 0.612198 | 9.463668 | 0.635104 |
| 8B 1 μM | 3.760656 | 0.045369 | 20.741240 | 0.022795 | 16.148254 | 0.711464 | 8.665186 | 0.959332 | 7.928790 | 0.951763 |
| Flavanone 20 μM | 7.669079 | 11.567501 | 13.151849 | 4.687945 | 18.376227 | |||||
| Control | 10.704364 | 0.538352 | 5.478535 | 0.005065 | 13.342202 | 0.946897 | 5.166537 | 0.570728 | 14.357457 | 0.008844 |
| 5B 20 μM | 4.570054 | 0.114164 | 10.405476 | 0.000000 | 12.298108 | 0.000260 | 6.868333 | 0.001660 | 1.315403 | 0.000000 |
| 6B 20 μM | 2.860606 | 0.001562 | 4.989167 | 0.000000 | 14.294118 | 0.009340 | 12.327883 | 0.000039 | 4.260733 | 0.009512 |
| 7B 20 μM | 4.570054 | 0.569024 | 14.867259 | 0.009041 | 11.607130 | 0.208327 | 2.927687 | 0.001286 | 8.586474 | 0.062300 |
| 8B 20 μM | 15.244568 | 0.912729 | 7.973248 | 0.011128 | 19.096473 | 0.332055 | 10.445151 | 0.000100 | 6.080636 | 0.346267 |
| Compound | Structure | R1 | R2 | R3 | R4 |
|---|---|---|---|---|---|
| 5B |  | H | CH3 | H | H |
| 6B | H | H | CH3 | H | |
| 7B | H | H | H | CH3 | |
| 8B | CH3 | H | H | H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłósek, M.; Krawczyk-Łebek, A.; Kostrzewa-Susłow, E.; Szliszka, E.; Bronikowska, J.; Jaworska, D.; Pietsz, G.; Czuba, Z.P. In Vitro Anti-Inflammatory Activity of Methyl Derivatives of Flavanone. Molecules 2023, 28, 7837. https://doi.org/10.3390/molecules28237837
Kłósek M, Krawczyk-Łebek A, Kostrzewa-Susłow E, Szliszka E, Bronikowska J, Jaworska D, Pietsz G, Czuba ZP. In Vitro Anti-Inflammatory Activity of Methyl Derivatives of Flavanone. Molecules. 2023; 28(23):7837. https://doi.org/10.3390/molecules28237837
Chicago/Turabian StyleKłósek, Małgorzata, Agnieszka Krawczyk-Łebek, Edyta Kostrzewa-Susłow, Ewelina Szliszka, Joanna Bronikowska, Dagmara Jaworska, Grażyna Pietsz, and Zenon P. Czuba. 2023. "In Vitro Anti-Inflammatory Activity of Methyl Derivatives of Flavanone" Molecules 28, no. 23: 7837. https://doi.org/10.3390/molecules28237837
APA StyleKłósek, M., Krawczyk-Łebek, A., Kostrzewa-Susłow, E., Szliszka, E., Bronikowska, J., Jaworska, D., Pietsz, G., & Czuba, Z. P. (2023). In Vitro Anti-Inflammatory Activity of Methyl Derivatives of Flavanone. Molecules, 28(23), 7837. https://doi.org/10.3390/molecules28237837