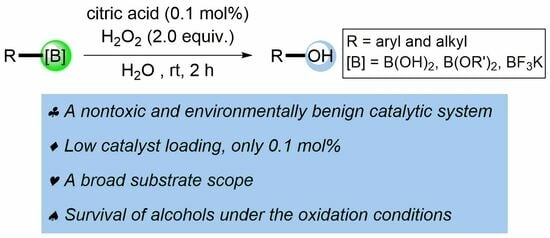
A Facile and General Oxidative Hydroxylation of Organoboron Compounds: Citric Acid as an Efficient Catalyst in Water to Access Phenolic and Alcoholic Motifs
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
3.2. Synthetic Procedures
3.2.1. The Typical Procedure for the Oxidative Hydroxylation of Arylboronic Acids
- phenol (2a): Yield: 98%, 92.2 mg, light pink solid, mp 38–40 °C, Rf = 0.50 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.29 (d, J = 6.2 Hz, 2H), 6.97 (t, J = 7.4 Hz, 1H), 6.87 (d, J = 7.7 Hz, 2H), and 4.64 (brs, 1H). 13C NMR (126 MHz, CDCl3) δ 155.5, 129.7 (2C), 120.8, and 115.3 (2C). [M + H]+ calcd for C6H7O, 95.0497; found 95.0493.
- p-cresol (2b): Yield: 98%, 105.7 mg, light brown solid, mp 30–32 °C, Rf = 0.52 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.06 (d, J = 8.0 Hz, 2H), 6.76 (d, J = 8.2 Hz, 2H), and 4.72 (brs, 1H), 2.30 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 153.2, 131.0, 130.1 (2C), and 115.1 (2C), 20.5. [M + H]+ calcd for C7H9O, 109.0653; found 109.0659.
- 3,5-dimethylphenol (2c): Yield: 97%, 119.6 mg, yellow solid, mp 59–61 °C, Rf = 0.62 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 6.64 (s, 1H), 6.52 (s, 2H), 5.12 (brs, 1H), and 2.31 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 155.3, 139.6 (2C), 122.6, 113.1 (2C), and 21.3 (2C). [M + H]+ calcd for C8H11O, 123.0810; found 123.0808.
- [1,1′-biphenyl]-4-ol (2d): Yield: 98%, 166.6 mg, light yellow solid, mp 162–164 °C, Rf = 0.44 (H/E = 5:1).1H NMR (500 MHz, CDCl3) δ 7.66–7.33 (m, 7H), 6.94 (d, J = 6.7 Hz, 2H), and 4.87 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 155.1, 140.8, 134.0, 131.0, 128.8 (2C), 128.4 (2C), 126.7 (2C), and 115.7(2C). [M + H]+ calcd for C12H11O, 171.0810; found 171.0815.
- [1,1′-biphenyl]-2-ol (2e): Yield: 99%, 168.8 mg, light yellow solid, mp 29–31 °C, Rf = 0.43 (H/E = 5:1).1H NMR (500 MHz, CDCl3) δ 7.56 (d, J = 4.4 Hz, 4H), 7.48–7.45 (m, 1H), 7.34 (d, J = 7.4 Hz, 2H), 7.08 (dd, J = 12.2, 7.4 Hz, 2H), and 5.40 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 152.5, 137.2, 130.4, 129.32 (2C), 129.25, 129.2, 128.3, 127.9 (2C), 121.0, and 116.0. [M + H]+ calcd for C12H11O, 171.0810; found 171.0818.
- 4-(methylthio)phenol (2f): Yield: 61%, 85.6 mg, white solid, mp 82–84 °C, Rf = 0.36 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.24 (d, J = 8.6 Hz, 2H), 6.81 (d, J = 8.6 Hz, 2H), 5.34 (brs, 1H), and 2.46 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 154.1, 130.4 (2C), 128.8, 116.1 (2C), and 18.1. [M + H]+ calcd for C7H9OS, 141.0374; found 141.0369.
- 2-methoxyphenol (2g): Yield: 93%, 115.2 mg, colorless oil, Rf = 0.62 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 6.97–6.95 (m, 1H), 6.92–6.88 (m, 3H), 5.67 (brs, 1H), and 3.92 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 146.6, 145.7, 121.5, 120.2, 114.6, 110.7, and 55.9. [M + H]+ calcd for C7H9O2, 125.0603; found 125.0605.
- 4-(heptyloxy)phenol (2h): Yield: 94%, 195.6 mg, white solid, mp 56–58 °C, Rf = 0.58 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 6.80 (q, J = 9.2 Hz, 4H), 5.34 (brs, 1H), 3.93 (t, J = 6.7 Hz, 2H), 1.79 (p, J = 6.9 Hz, 2H), 1.53–1.42 (m, 2H), 1.43–1.28 (m, 6H), and 0.93 (t, J = 6.7 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 153.2, 149.4, 116.1 (2C), 115.8 (2C), 69.0, 31.8, 29.4, 29.1, 26.0, 22.6, and 14.1. [M + H]+ calcd for C13H21O2, 209.1542; found 209.1540.
- 4-phenoxyphenol (2i): Yield: 94%, 175.0 mg, light brown solid, mp 74–76 °C, Rf = 0.48 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.34 (t, J = 7.9 Hz, 2H), 7.09 (t, J = 7.3 Hz, 1H), 7.00–6.97 (m, 4H), 6.86 (d, J = 8.8 Hz, 2H), and 5.28 (brs, 1H). 13C NMR (126 MHz, CDCl3) δ 158.4, 151.7, 150.3, 129.7 (2C), 122.7, 121.1 (2C), 117.7 (2C), and 116.5 (2C). [M + H]+ calcd for C12H11O2, 187.0759; found 187.0759.
- 4-(diphenylamino)phenol (2j): Yield: 78%, 204.2 mg, white solid, mp 118–120 °C, Rf = 0.48 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.26 (t, J = 8.6 Hz, 4H), 7.13–6.97 (m, 8H), 6.81 (d, J = 8.1 Hz, 2H), and 4.98 (brs, 1H). 13C NMR (126 MHz, CDCl3) δ 152.1, 148.2(2C), 141.0, 129.1 (4C), 127.6 (2C), 123.0 (4C), 122.0 (2C), and 116.3 (2C). [M + H]+ calcd for C18H16NO, 262.1232; found 262.1237.
- benzo[d][1,3]dioxol-5-ol (2k): Yield: 91%, 125.6 mg, white solid, mp 58–60 °C, Rf = 0.38 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 6.68 (d, J = 8.3 Hz, 1H), 6.45 (d, J = 2.4 Hz, 1H), 6.28 (dd, J = 8.3, 2.4 Hz, 1H), 5.93 (s, 2H), and 4.87 (brs, 1H). 13C NMR (126 MHz, CDCl3) δ 150.5, 148.3, 141.6, 108.2, 106.8, 101.2, and 98.4. [M + H]+ calcd for C7H7O3, 139.0395; found 139.0393.
- 3-chlorophenol (2l): Yield: 98%, 125.8 mg, colorless oil, Rf = 0.51 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.18 (t, J = 8.1 Hz, 1H), 6.94 (d, J = 7.9 Hz, 1H), 6.88 (s, 1H), 6.76–6.74 (m, 1H), and 5.11 (brs, 1H). 13C NMR (126 MHz, CDCl3) δ 156.3, 134.9, 130.5, 121.1, 115.9, and 113.8. [M + H]+ calcd for C6H6ClO, 129.0107; found 129.0111.
- 1-(3-hydroxyphenyl)ethan-1-one (2m): Yield: 94%, 127.8 mg, yellow solid, mp 92–94 °C, Rf = 0.54 = (H/E 5:1). 1H NMR (500 MHz, CDCl3) δ 7.58 (s, 1H), 7.53 (d, J = 7.7 Hz, 1H), 7.36 (t, J = 7.9 Hz, 1H), 7.15 (dd, J = 8.1, 2.5 Hz, 1H), 6.98 (brs, 1H), and 2.63 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 199.7, 156.5, 138.3, 130.0, 121.1, 121.0, 114.8, and 26.8. [M + H]+ calcd for C8H9O2, 137.0603; found 137.0599.
- 4-hydroxybenzonitrile (2n): Yield: 91%, 108.3 mg, gray solid, mp 110–112 °C, Rf = 0.20 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.58 (d, J = 8.7 Hz, 2H), 7.22 (brs, 1H), and 6.98 (d, J = 8.8 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 160.5, 134.4 (2C), 119.3, 116.6 (2C), and 102.8. [M + H]+ calcd for C7H6NO, 120.0449; found 120.0457.
- 4-nitrophenol (2o): Yield: 80%, 111.3 mg, yellow solid, mp 108–110 °C, Rf = 0.18 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 8.20 (d, J = 8.8 Hz, 2H), 6.96 (d, J = 8.8 Hz, 2H), and 6.14 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 161.5, 141.6, 126.3 (2C), and 115.8 (2C). [M + H]+ calcd for C6H6NO3, 140.0348; found 140.0349.
- naphthalen-2-ol (2p): Yield: 98%, 141.2 mg, white solid, mp 118–120 °C, Rf = 0.48 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.83–7.79 (m, 2H), 7.71 (d, J = 7.9 Hz, 1H), 7.48 (t, J = 7.1 Hz, 1H), 7.38 (t, J = 7.0 Hz, 1H), 7.18–7.14 (m, 2H), and 5.20 (brs, 1H). 13C NMR (126 MHz, CDCl3) δ 153.3, 134.6, 123.0, 129.0, 127.8, 126.6, 126.5, 123.7, 117.8, and 109.6. [M + H]+ calcd for C10H9O, 145.0653; found 145.0658.
- naphthalen-1-ol (2q): Yield: 95%, 136.8 mg, white solid, mp 92–94 °C, Rf = 0.52 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 8.24–8.22 (m, 1H), 7.88–7.86 (m, 1H), 7.56–7.49 (m, 3H), 7.35 (t, J = 7.8 Hz, 1H), 6.85 (d, J = 7.4 Hz, 1H), and 5.35 (brs, 1H). 13C NMR (126 MHz, CDCl3) δ 151.4, 134.8, 127.7, 126.5, 125.9, 125.3, 124.4, 121.6, 120.8, and 108.7. [M + H]+ calcd for C10H9O, 145.0653; found 145.0655.
- 6-methoxynaphthalen-2-ol (2r): Yield: 90%, 156.8 mg, white solid, mp 146–148 °C, Rf = 0.56 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.67 (d, J = 8.7 Hz, 1H), 7.61 (d, J = 8.8 Hz, 1H), 7.16–7.09 (m, 4H), 4.90 (s, 1H), and 3.93 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 156.1, 151.8, 129.8, 129.7, 128.5, 127.8, 119.3, 118.1, 109.8, 106.0, and 55.3. [M + H]+ calcd for C11H11O2, 175.0759; found 175.0766.
- 4-(9H-carbazol-9-yl)phenol (2s): Yield: 78%, 202.3 mg, white solid, mp 94–96 °C, Rf = 0.40 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 8.25 (t, J = 7.2 Hz, 2H), 7.52–7.36 (m, 8H), 7.06 (d, J = 8.4 Hz, 2H), and 5.26 (brs, 1H). 13C NMR (126 MHz, CDCl3) δ 154.8, 141.4 (2C), 130.6, 128.9 (2C), 126.0 (2C), 123.2 (2C), 120.4 (2C), 119.9 (2C), 116.7 (2C), and 109.8 (2C). [M + H]+ calcd for C18H14NO, 260.1075; found 260.1071.
- 4-((1s,4r)-4-propylcyclohexyl)phenol (2t): Yield: 98%, 214.1 mg, white solid, mp 44–46 °C, Rf = 0.42 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.10 (d, J = 8.5 Hz, 2H), 6.78 (d, J = 8.5 Hz, 2H), 4.69 (brs, 1H), 2.45–2.40 (m, 1H), 1.89–1.86 (m, 4H), 1.47–1.21 (m, 7H), 1.10–1.02 (m, 2H), and 0.93 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 153.5, 140.4, 127.9 (2C), 115.0 (2C), 43.8, 39.8, 37.0, 34.6 (2C), 33.6 (2C), 20.1, and 14.4. [M + H]+ calcd for C15H23O, 219.1749; found 219.1754.
3.2.2. The Typical Procedure for the Oxidative Hydroxylation of Alkylboronic Acids
- 2-phenylethan-1-ol (4a): Yield: 90%, 110.0 mg, colorless oil, Rf = 0.40 (H/E = 5:1). 1H NMR (500 MHz, CDCl3) δ 7.35 (t, J = 7.7 Hz, 2H), 7.28–7.25 (m, 3H), 3.89 (t, J = 6.6 Hz, 2H), 2.90 (t, J = 6.5 Hz, 2H), and 1.54 (brs, 1H). 13C NMR (126 MHz, CDCl3) δ 138.5, 129.1 (2C), 128.6 (2C), 126.5, 63.7, and 39.2. [M + H]+ calcd for C8H11O, 123.0810; found 123.0807.
- cyclohexanol (4b): Yield: 88%, 88.2 mg, colorless oil, Rf = 0.34 (H/E = 4:1). 1H NMR (600 MHz, CDCl3) δ 3.53–3.50 (m, 1H), 2.94 (s, 1H), 1.82 (dd, J = 9.6, 4.8 Hz, 2H), 1.67 (dd, J = 9.1, 4.7 Hz, 2H), 1.50–1.46 (m, 1H), 1.22–1.16 (m, 4H), and 1.12–1.07 (m, 1H). 13C NMR (151 MHz, CDCl3) δ 70.1, 35.4 (2C), 25.4, and 24.2 (2C). [M + H]+ calcd for C6H13O, 101.0966; found 101.0967.
- hexan-1-ol (4c): Yield: 84%, 85.8 mg, colorless oil, Rf = 0.38 (H/E = 4:1). 1H NMR (600 MHz, CDCl3) δ 4.41 (brs, 1H), 3.30 (t, J = 6.9 Hz, 2H), 1.29 (p, J = 6.9 Hz, 2H), 1.22–0.95 (m, 6H), and 0.65 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 61.8, 32.3, 31.5, 25.3, 22.4, and 13.6. [M + H]+ calcd for C6H15O, 103.1123; found 103.1128.
- heptan-1-ol (4d): Yield: 84%, 97.6 mg, colorless oil, Rf = 0.40 (H/E = 4:1). 1H NMR (600 MHz, CDCl3) δ 3.64 (t, J = 6.7 Hz, 2H), 1.68 (s, 1H), 1.62–1.53 (m, 2H), 1.38–1.26 (m, 8H), and 0.90 (t, J = 7.0 Hz, 3H).13C NMR (151 MHz, CDCl3) δ 63.0, 32.8, 31.8, 29.1, 25.7, 22.6, and 14.1. [M + H]+ calcd for C7H17O, 117.1279; found 117.1277.
- decan-1-ol (4e): Yield: 90%, 142.4 mg, colorless oil, Rf = 0.48 (H/E = 4:1). 1H NMR (600 MHz, CDCl3) δ 3.64 (t, J = 6.7 Hz, 2H), 1.68 (s, 1H), 1.61–1.53 (m, 2H), 1.38–1.25 (m, 14H), and 0.89 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 63.0, 32.8, 31.9, 29.64, 29.57, 29.5, 29.3, 25.8, 22.7, and 14.1. [M + H]+ calcd for C10H23O, 159.1749; found 159.1752.
3.2.3. Substrate Extension Studies
3.3. Large-Scale Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rappoport, Z. The Chemistry of Phenols; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Cox, P.B.; Njardarson, J.T. Phenols in Pharmaceuticals: Analysis of a Recurring Motif. J. Med. Chem. 2022, 65, 7044–7072. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, A.; Savoini, A.; Saporetti, R.; Franchi, P.; Lucarini, M.; Cozzi, P.G. A facile hydroxylation of arylboronic acids mediated by sodium ascorbate. Org. Chem. Front. 2018, 5, 1573–1578. [Google Scholar] [CrossRef]
- Yin, W.; Pan, X.; Leng, W.; Chen, J.; He, H. The highly efficient air oxidation of aryl and alkyl boronic acids by a microwave-assisted protocol under transition metal-free conditions. Green Chem. 2019, 21, 4614–4618. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, C.; Zheng, Y.; Zhang, X. Isotruxene-based porous polymers as efficient and recyclable photocatalysts for visible-light induced metal-free oxidative organic transformations. Green Chem. 2021, 23, 8878–8885. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Goddard, V.H.M.; Schubert, C.P.J.; Gopee, H.; Hughes, D.L.; Gonzalez-Lucas, D. Unexpected Phenol Production from Arylboronic Acids under Palladium-Free Conditions; Organocatalyzed Air Oxidation. Org. Lett. 2011, 13, 6034. [Google Scholar] [CrossRef]
- Kaewmati, P.; Somsook, E.; Dhital, R.N.; Sakurai, H. Aerobic oxygenation of phenylboronic acid promoted by thiol derivatives under gold-free conditions: A warning against gold nanoparticle catalysis. Tetrahedron Lett. 2012, 53, 6104–6106. [Google Scholar] [CrossRef]
- Hosoi, K.; Kuriyama, Y.; Inagi, S.; Fuchigami, T. Electrochemical hydroxylation of organoboron compounds. Chem. Commun. 2010, 46, 1284–1286. [Google Scholar] [CrossRef]
- Jiang, H.; Lykke, L.; Pedersen, S.U.; Xiao, W.-J.; Jørgensen, K.A. A practical electromediated ipso-hydroxylation of aryl and alkyl boronic acids under an air atmosphere. Chem. Commun. 2012, 48, 7203–7205. [Google Scholar] [CrossRef]
- Simon, J.; Salzbrunn, S.; Prakash, G.K.S.; Petasis, N.A.; Olah, G.A. Regioselective Conversion of Arylboronic Acids to Phenols and Subsequent Coupling to Symmetrical Diaryl Ethers. J. Org. Chem. 2001, 66, 633–634. [Google Scholar] [CrossRef]
- Kwon, G.-T.; Kim, S.-H. Tylenol® and Aspirin® as Green Promoters for Ipso-Hydroxylation of Arylboronic Acids. Lett. Org. Chem. 2022, 19, 902–907. [Google Scholar] [CrossRef]
- Mahanta, A.; Adhikari, P.; Bora, U.; Thakur, A.J. Biosilica as an efficient heterogeneous catalyst for ipso-hydroxylation of arylboronic acids. Tetrahedron Lett. 2015, 56, 1780–1783. [Google Scholar] [CrossRef]
- Das, S.K.; Bhattacharjee, P.; Bora, U. Ascorbic Acid as a Highly Efficient Organocatalyst for ipso-Hydroxylation of Arylboronic Acid. ChemistrySelect 2018, 3, 2131–2134. [Google Scholar] [CrossRef]
- Phukan, S.; Mahanta, A.; Rashid, M.H. Size-tunable ZnO nanotapes as an efficient catalyst for oxidative chemoselective C-B bond cleavage of arylboronic acids. Appl. Catal. A-Gen. 2018, 562, 58–66. [Google Scholar] [CrossRef]
- Agarwal, S.; Deori, K. Copper Sulfide Nanosheets for Photocatalytic Oxidation of Benzyl Alcohols and Hydroxylation of Arylboronic Acids. ACS Appl. Nano Mater. 2022, 5, 4413–4422. [Google Scholar] [CrossRef]
- Begum, T.; Gogoi, A.; Gogoi, P.K.; Bora, U. Catalysis by mont K-10 supported silver nanoparticles: A rapid and green protocol for the efficient ipso-hydroxylation of arylboronic acids. Tetrahedron Lett. 2015, 56, 95–97. [Google Scholar] [CrossRef]
- Shin, E.-J.; Kim, H.-S.; Joo, S.-R.; Shin, U.S.; Kim, S.-H. Heterogeneous Palladium–Chitosan–CNT Core–Shell Nanohybrid Composite for Ipso-hydroxylation of Arylboronic Acids. Catal. Lett. 2019, 149, 1560–1564. [Google Scholar] [CrossRef]
- Guo, S.; Lu, L.; Cai, H. Base-Promoted, Mild and Highly Efficient Conversion of Arylboronic Acids into Phenols with tert-Butyl Hydroperoxide. Synlett 2013, 24, 1712–1714. [Google Scholar] [CrossRef]
- Chen, D.-S.; Huang, J.-M. A Mild and Highly Efficient Conversion of Arylboronic Acids into Phenols by Oxidation with MCPBA. Synlett 2013, 24, 499–501. [Google Scholar] [CrossRef]
- Kianmehr, E.; Yahyaee, M.; Tabatabai, K. A mild conversion of arylboronic acids and their pinacolyl boronate esters into phenols using hydroxylamine. Tetrahedron Lett. 2007, 48, 2713–2715. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, R.; Falck, J.R. Mild and Rapid Hydroxylation of Aryl/Heteroaryl Boronic Acids and Boronate Esters with N-Oxides. Org. Lett. 2012, 14, 3494–3497. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Chowdhury, H.; Sneh, K.; Goswami, A. Hydroxylation of aryl- and alkylboronic acids/esters mediated by iodobenzene diacetate—An avenue for using organoboronic acids/esters as nucleophiles for hydroxylation reactions. Tetrahedron Lett. 2015, 56, 172–174. [Google Scholar] [CrossRef]
- Gogoi, P.; Bezboruah, P.; Gogoi, J.; Boruah, R.C. ipso-Hydroxylation of Arylboronic Acids and Boronate Esters by Using Sodium Chlorite as an Oxidant in Water. Eur. J. Org. Chem. 2013, 2013, 7291–7294. [Google Scholar] [CrossRef]
- Gogoi, A.; Bora, U. An Iodine-Promoted, Mild and Efficient Method for the Synthesis of Phenols from Arylboronic Acids. Synlett 2012, 23, 1079–1081. [Google Scholar]
- Prakash, G.K.S.; Chacko, S.; Panja, C.; Thomas, T.E.; Gurung, L.; Rasul, G.; Mathew, T.; Olah, G.A. Regioselective Synthesis of Phenols and Halophenols from Arylboronic Acids Using Solid Poly(N-vinylpyrrolidone)/ Hydrogen Peroxide and Poly(4-vinylpyridine)/Hydrogen Peroxide Complexes. Adv. Synth. Catal. 2009, 351, 1567–1574. [Google Scholar] [CrossRef]
- Mulakayala, N.; Kumar, K.M.; Rapolu, R.K.; Kandagatla, B.; Rao, P.; Oruganti, S.; Pal, M. Catalysis by Amberlite IR-120 resin: A rapid and green method for the synthesis of phenols from arylboronic acids under metal, ligand, and base-free conditions. Tetrahedron Lett. 2012, 53, 6004–6007. [Google Scholar] [CrossRef]
- Gohain, M.; Plessis, M.; van Tonder, J.H.; Bezuidenhoudt, B.C.B. Preparation of phenolic compounds through catalyst-free ipso-hydroxylation of arylboronic acids. Tetrahedron Lett. 2014, 55, 2082–2084. [Google Scholar] [CrossRef]
- Wagh, R.B.; Nagarkar, J.M. Silica chloride: An efficient promoter for oxidation of arylboronic acids to phenols. Tetrahedron Lett. 2017, 58, 3323–3326. [Google Scholar] [CrossRef]
- Karthik, M.; Suresh, P. Graphene Oxide as a Carbocatalyst for Sustainable ipso-Hydroxylation of Arylboronic Acids: A Simple and Straightforward Strategy To Access Phenols. ACS Sustain. Chem. Eng. 2019, 7, 9028–9034. [Google Scholar] [CrossRef]
- Choudhary, P.; Kumari, K.; Sharma, D.; Kumar, S.; Krishnan, V. Surface Nanoarchitectonics of Boron Nitride Nanosheets for Highly Efficient and Sustainable ipso-Hydroxylation of Arylboronic Acids. ACS Appl. Mater. Interfaces 2023, 15, 9412–9420. [Google Scholar] [CrossRef]
- Huang, W.-C.; Li, B.; Qi, X.; Mao, X. New type of green extractant for oil production: Citric acid/citric acid sodium extraction system. Food Chem. 2020, 310, 125815. [Google Scholar] [CrossRef] [PubMed]
- Zolfigol, M.A.; Mokhlesi, M.; Farahmand, S. Application of citric acid as highly efficient and green organocatalyst for multi-component synthesis of indazolo[2,1-b]phthalazine-triones. J. Iran. Chem. Soc. 2013, 10, 577–581. [Google Scholar] [CrossRef]
- Ding, Q.-S.; Zhang, J.-L.; Chen, J.-X.; Liu, M.-C.; Ding, J.-C.; Wu, H.-Y. Tandem synthesis of 2,3-dihydroquinazolin-4(1H)-ones on grinding under solvent-free conditions. J. Heterocycl. Chem. 2012, 49, 375–380. [Google Scholar] [CrossRef]
- Gundala, T.R.; Godugu, K.; Nallagondu, C.G.R. Citric Acid-catalyzed Synthesis of 2,4-Disubstituted Thiazoles from Ketones via C–Br, C–S, and C–N Bond Formations in One Pot: A Green Approach. J. Chin. Chem. Soc. 2017, 64, 1408–1416. [Google Scholar] [CrossRef]
- Gawande, M.B.; Brancoa, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef]




 | ||||
|---|---|---|---|---|
| Entry | Cat. (x mol) | Oxidant (x equiv.) | Temp. (°C) | Yield (%) b |
| 1 | citric acid (0.1) | H2O2 (2.0) | 40 | 93 |
| 2 | citric acid (0.1) | H2O2 (2.0) | 30 | 96 |
| 3 | citric acid (0.1) | H2O2 (2.0) | rt | 98 |
| 4 | citric acid (0.1) | air | rt | n. d. c |
| 5 | citric acid (0.1) | TBHP (2.0) | rt | 30 |
| 6 | citric acid (0.1) | DTBP (2.0) | rt | 40 |
| 7 | citric acid (0.1) | O2 (balloon) | rt | n. d. |
| 8 | citric acid (0.1) | NaClO2 (2.0) | rt | 64 |
| 9 | citric acid (0.1) | H2O2 (1.0) | rt | 68 |
| 10 | citric acid (0.1) | H2O2 (3.0) | rt | 95 |
| 11 | citric acid (0.05) | H2O2 (2.0) | rt | 90 |
| 12 | citric acid (0.2) | H2O2 (2.0) | rt | 97 |
| 13 d | citric acid (0.1) | H2O2 (2.0) | rt | 91 |
| 14 | - | H2O2 (2.0) | rt | 66 |
| 15 | acetic acid (0.1) | H2O2 (2.0) | rt | 69 |
| 16 | benzoic acid (0.1) | H2O2 (2.0) | rt | 74 |
| 17 | tartaric acid (0.1) | H2O2 (2.0) | rt | 83 |
| 18 | formic acid (0.1) | H2O2 (2.0) | rt | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.-H.; Chen, X.; Yang, D.; Liu, C.-Y.; Zhou, X.-Y. A Facile and General Oxidative Hydroxylation of Organoboron Compounds: Citric Acid as an Efficient Catalyst in Water to Access Phenolic and Alcoholic Motifs. Molecules 2023, 28, 7915. https://doi.org/10.3390/molecules28237915
Zhou J-H, Chen X, Yang D, Liu C-Y, Zhou X-Y. A Facile and General Oxidative Hydroxylation of Organoboron Compounds: Citric Acid as an Efficient Catalyst in Water to Access Phenolic and Alcoholic Motifs. Molecules. 2023; 28(23):7915. https://doi.org/10.3390/molecules28237915
Chicago/Turabian StyleZhou, Jia-Hui, Xia Chen, Dan Yang, Chun-Yan Liu, and Xiao-Yu Zhou. 2023. "A Facile and General Oxidative Hydroxylation of Organoboron Compounds: Citric Acid as an Efficient Catalyst in Water to Access Phenolic and Alcoholic Motifs" Molecules 28, no. 23: 7915. https://doi.org/10.3390/molecules28237915
APA StyleZhou, J. -H., Chen, X., Yang, D., Liu, C. -Y., & Zhou, X. -Y. (2023). A Facile and General Oxidative Hydroxylation of Organoboron Compounds: Citric Acid as an Efficient Catalyst in Water to Access Phenolic and Alcoholic Motifs. Molecules, 28(23), 7915. https://doi.org/10.3390/molecules28237915