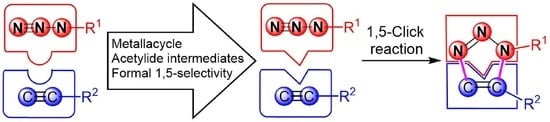
Overview of 1,5-Selective Click Reaction of Azides with Alkynes or Their Synthetic Equivalents
Abstract
:1. Introduction
2. 1,5-Selective Click Reaction of Azide with Alkyne via Metallacyclic Intermediates
2.1. Ruthenium-Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
2.1.1. Various Reaction Conditions of Ruthenium-Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
2.1.2. Mechanism of Ruthenium-Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
2.2. Nickel-Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
3. 1,5-Selective Click Reaction of Azide with Alkyne via Acetylide Intermediates
3.1. Transition-Metal-Free 1,5-Selective Click Reaction of Azide with Alkyne
3.2. Zinc-Mediated 1,5-Selective Click Reaction of Azide with Alkyne
3.3. Rare-Earth Metal Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
4. Formal 1,5-Selective Click Reaction of Azide with Alkyne
4.1. In Situ Generation of Terminal Acetylene
4.2. Formal 1,5-Selective Click Reaction via Cycloaddition/Elimination
4.2.1. Cycloaddition/Elimination of Sulfonyl Group
4.2.2. Cycloaddition/Elimination of HNO2/HOAc Group
4.2.3. Cycloaddition/Elimination of Protection Group
4.3. Formal 1,5-Selective Click Reaction of Azide with Alkyne via Wittig Reaction
4.4. Other Formal 1,5-Selective Click Reaction of Azide with Alkyne
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Prize Announcement. Available online: https://www.nobelprize.org/prizes/chemistry/2022/summary/ (accessed on 3 January 2023).
- Abd-Elaal, A.A.; Aiad, I.; Shaban, S.M.; Tawfik, S.M.; Sayed, A. Synthesis and evaluation of some triazole derivatives as corrosion inhibitors and biocides. J. Surfactants Deterg. 2014, 17, 483–491. [Google Scholar] [CrossRef]
- Duan, T.; Fan, K.; Fu, Y.; Zhong, C.; Chen, X.; Peng, T.; Qin, J. Triphenylamine-based organic dyes containing a 1,2,3-triazole bridge for dye-sensitized solar cells via a ‘Click’ reaction. Dyes Pigm. 2012, 94, 28–33. [Google Scholar] [CrossRef]
- Abu-Orabi, S.T.; Atfah, M.A.; Jibril, I.; Mari’i, F.M.; Ali, A.A.-S. Dipolar cycloaddition reactions of organic azides with some acetylenic-compounds. J. Heterocycl. Chem. 1989, 26, 1461–1468. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Li, X.; Sun, Y.; Zhao, Y.; Jia, S.; Guo, N.; Xu, G.; Zhang, W. Copper(II) acetylacetonate: An efficient catalyst for Huisgen-click reaction for synthesis of 1,2,3-triazoles in water. Chin. J. Chem. 2017, 35, 1239–1245. [Google Scholar] [CrossRef]
- Shi, S.; Wang, Z.; Deng, Y.; Tian, F.; Wu, Q.; Zheng, P. Combination of click chemistry and enzymatic ligation for stable and efficient protein immobilization for single-molecule force spectroscopy. CCS Chem. 2022, 4, 598–604. [Google Scholar] [CrossRef]
- Totobenazara, J.; Burke, A.J. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Jalani, H.B.; Karagöz, A.C.; Tsogoeva, S.B. Synthesis of substituted 1,2,3-triazoles via metal-free click cycloaddition reactions and alternative cyclization methods. Synthesis 2017, 49, 29–41. [Google Scholar]
- Huisgen, R. 1,3-Dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Hashjin, M.C.; Ciyabi, R.; Baharloui, M.; Hosseini, G.; Tavakoli, H. Copper supported on the SiO2 nanoparticle in click chemistry: An alternative catalytic system for regioselective and one-pot synthesis of 1,2,3-triazoles and β-hydroxytriazoles. Chin. J. Chem. 2012, 30, 223–227. [Google Scholar] [CrossRef]
- Albadi, J.; Keshavarz, M.; Abedini, M.; Vafaie-nezhad, M. Copper iodide nanoparticles on poly(4-vinyl pyridine) as new and green catalyst for multicomponent click synthesis of 1,4-disubstituted-1,2,3-triazoles in water. Chin. Chem. Lett. 2012, 23, 797–800. [Google Scholar] [CrossRef]
- Friscourt, F.; Boons, G.J. One-pot three-step synthesis of 1,2,3-triazoles by copper-catalyzed cycloaddition of azides with alkynes formed by a sonogashira cross-coupling and desilylation. Org. Lett. 2010, 12, 4936–4939. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R. Nitroacetylene as dipolarophile in [2+3] cycloaddition reactions with allenyl-type three-atom components: DFT computational study. Monatsh. Chem. 2015, 146, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ikhlef, D.; Kahlal, S.; Saillard, J.Y.; Astruc, D. Metal-catalyzed azide-alkyne "click" reactions: Mechanistic overview and recent trends. Coord. Chem. Rev. 2016, 316, 1–20. [Google Scholar] [CrossRef]
- Gomes, R.S.; Jardim, G.A.M.; de Carvalho, R.L.; Araujo, M.H.; da Silva, E.N. Beyond copper-catalyzed azide-alkyne 1,3-dipolar cycloaddition: Synthesis and mechanism insights. Tetrahedron 2019, 75, 3697–3712. [Google Scholar] [CrossRef]
- Johansson, J.R.; Beke-Somfai, T.; Stålsmeden, A.S.; Kann, N. Ruthenium-catalyzed azide alkyne cycloaddition reaction: Scope, mechanism, and applications. Chem. Rev. 2016, 116, 14726–14768. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of azide-alkyne cycloaddition-click chemistry over the recent decade. Tetrahedron 2016, 72, 5257–5283. [Google Scholar] [CrossRef]
- Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-Triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions. Eur. J. Org. Chem. 2014, 2014, 3289–3306. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, G.; Song, J.; Ren, H. Recent developments in azide-free synthesis of 1,2,3-triazoles. Chin. J. Chem. 2017, 35, 1797–1807. [Google Scholar] [CrossRef]
- Agrahari, A.K.; Bose, P.; Jaiswal, M.K.; Rajkhowa, S.; Singh, A.S.; Hotha, S.; Mishra, N.; Tiwari, V.K. Cu(I)-catalyzed click chemistry in glycoscience and their diverse applications. Chem. Rev. 2021, 121, 7638–7955. [Google Scholar] [CrossRef] [PubMed]
- Opsomer, T.; Dehaen, W. Metal-free syntheses of N-functionalized and NH-1,2,3-triazoles: An update on recent developments. Chem. Commun. 2021, 57, 1568–1590. [Google Scholar] [CrossRef] [PubMed]
- Oppilliart, S.; Mousseau, G.; Zhang, L.; Jia, G.; Thuery, P.; Rousseau, B.; Cintrat, J.C. 1-Protected 5-amido 1,2,3-triazoles via ruthenium-catalyzed [3+2] cycloaddition of azides and ynamides. Tetrahedron 2007, 63, 8094–8098. [Google Scholar] [CrossRef]
- Nulwala, H.; Takizawa, K.; Odukale, A.; Khan, A.; Thibault, R.J.; Taft, B.R.; Lipshutz, B.H.; Hawker, C.J. Synthesis and characterization of isomeric vinyl-1,2,3-triazole materials by azide-alkyne click chemistry. Macromolecules 2009, 42, 6068–6074. [Google Scholar] [CrossRef]
- Wuest, F.; Tang, X.; Kniess, T.; Pietzsch, J.; Suresh, M. Synthesis and cyclooxygenase inhibition of various (aryl-1,2,3-triazole-1-yl)-methanesulfonylphenyl derivatives. Bioorg. Med. Chem. 2009, 17, 1146–1151. [Google Scholar] [CrossRef]
- Wang, D.; Salmon, L.; Ruiz, J.; Astruc, D. A recyclable ruthenium(II) complex supported on magnetic nanoparticles: A regioselective catalyst for alkyne-azide cycloaddition. Chem. Commun. 2013, 49, 6956–6958. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.R.; Lincoln, P.; Nordén, B.; Kann, N. Sequential one-pot ruthenium-catalyzed azide-alkyne cycloaddition from primary alkyl halides and sodium azide. J. Org. Chem. 2011, 76, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.K.; Boren, B.C.; Fokin, V.V. Ruthenium-catalyzed cycloaddition of aryl azides and alkynes. Org. Lett. 2007, 9, 5337–5339. [Google Scholar] [CrossRef]
- Boren, B.C.; Narayan, S.; Rasmussen, L.K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V.V. Ruthenium-catalyzed azide-alkyne cycloaddition: Scope and mechanism. J. Am. Chem. Soc. 2008, 130, 8923–8930. [Google Scholar] [CrossRef]
- Johansson, J.R.; Hermansson, E.; Nordén, B.; Kann, N.; Beke-Somfai, T. Peptides from RuAAC-derived 1,5-disubstituted triazole units. Eur. J. Org. Chem. 2014, 2014, 2703–2713. [Google Scholar] [CrossRef]
- Stålsmeden, A.S.; Paterson, A.J.; Szigyártó, I.C.; Thunberg, L.; Johansson, J.R.; Beke-Somfai, T.; Kann, N. Chiral 1,5-disubstituted 1,2,3-triazoles—Versatile tools for foldamers and peptidomimetic applications. Org. Biomol. Chem. 2020, 18, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-H.; Wu, F.-L.; Chiang, G.-R.; He, S.-T.; Lo, Y.-H. Preparation of ruthenium azido complex containing a Tp ligand and ruthenium-catalyzed cycloaddition of organic azides with alkynes in organic and aqueous media: Experimental and computational studies. J. Organomet. Chem. 2014, 774, 57–60. [Google Scholar] [CrossRef]
- Lamberti, M.; Fortman, G.C.; Poater, A.; Broggi, J.; Slawin, A.M.Z.; Cavallo, L.; Nolan, S.P. Coordinatively unsaturated ruthenium complexes as efficient alkyne-azide cycloaddition catalysts. Organometallics 2012, 31, 756–767. [Google Scholar] [CrossRef]
- Kim, W.G.; Kang, M.E.; Lee, J.B.; Jeon, M.H.; Lee, S.; Lee, J.; Cho, B.; Cal, P.; Kang, S.; Kee, J.M.; et al. Nickel-catalyzed azide-alkyne cycloaddition to access 1,5-disubstituted 1,2,3-triazoles in air and water. J. Am. Chem. Soc. 2017, 139, 12121–12124. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.G.; Baek, S.Y.; Jeong, S.Y.; Nam, D.; Jeon, J.H.; Choe, W.; Baik, M.H.; Hong, S.Y. Chemo- and regioselective click reactions through nickel-catalyzed azide-alkyne cycloaddition. Org. Biomol. Chem. 2020, 18, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Akimova, G.; Chistokletov, V.; Petrov, A. 1,3-dipolar addition to unsaturated compounds XVII. The reaction of azides with Iotsich complexes obtained from phenyl- and alkenylacetylenes. Zh. Obshch. Khim. 1967, 3, 968–974. [Google Scholar]
- Krasinski, A.; Fokin, V.V.; Sharpless, K.B. Direct synthesis of 1,5-disubstituted-4-magnesio-1,2,3-triazoles, revisited. Org. Lett. 2004, 6, 1237–1240. [Google Scholar] [CrossRef]
- Banday, A.; Hruby, V. Regioselective N/C-heterocyclization of allenylindium bromide across aryl azides: One-pot synthesis of 5-methyl-1,2,3-triazoles. Synlett 2014, 25, 1859–1862. [Google Scholar] [CrossRef]
- Kwok, S.W.; Fotsing, J.R.; Fraser, R.J.; Rodionov, V.O.; Fokin, V.V. Transition-metal-free catalytic synthesis of 1,5-diaryl-1,2,3-triazoles. Org. Lett. 2010, 12, 4217–4219. [Google Scholar] [CrossRef]
- Smith, C.D.; Greaney, M.F. Zinc mediated azide-alkyne ligation to 1,5-and 1,4,5-substituted 1,2,3-triazoles. Org. Lett. 2013, 15, 4826–4829. [Google Scholar] [CrossRef]
- Li, Y.; Qi, X.; Lei, Y.; Lan, Y. Mechanism and selectivity for zinc-mediated cycloaddition of azides with alkynes: A computational study. RSC Adv. 2015, 5, 49802–49808. [Google Scholar] [CrossRef]
- Liu, B.; Cui, D. Rare-earth metal complexes stabilized by amino-phosphine ligand. Reaction with mesityl azide and catalysis of the cycloaddition of organic azides and aromatic alkynes. Dalton Trans. 2009, 3, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Lin, W.; Zhang, F.; Liu, R.; Zhou, X. Ln[N(SiMe3)2]3-catalyzed cycloaddition of terminal alkynes to azides leading to 1,5-disubstituted 1,2,3-triazoles: New mechanistic features. Chem. Commun. 2013, 49, 5589–5591. [Google Scholar] [CrossRef]
- Wang, J.-M.; Yu, S.-B.; Li, Z.-M.; Wang, Q.-R.; Li, Z.-T. Mechanism of samarium-catalyzed 1,5-regioselective azide-alkyne [3+2]-cycloaddition: A quantum mechanical investigation. J. Phys. Chem. A 2015, 119, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, Y.; Luo, J.; Sun, Q.; Peng, M.; Lin, Q. Base-mediated reaction of vinyl bromides with aryl azides: One-pot synthesis of 1,5-disubstituted 1,2,3-triazoles. Tetrahedron Lett. 2014, 55, 3847–3850. [Google Scholar] [CrossRef]
- Zhang, X.; Rakesh, K.P.; Qin, H. Transition-metal-free regioselective construction of 1,5-diaryl-1,2,3-triazoles through dehydrative cycloaddition of alcohols with aryl azides mediated by SO2F2. Chem. Commun. 2019, 55, 2845–2848. [Google Scholar] [CrossRef]
- Zha, G.; Fang, W.; Li, Y.; Leng, J.; Chen, X.; Qin, H.L. SO2F2-mediated oxidative dehydrogenation and dehydration of alcohols to alkynes. J. Am. Chem. Soc. 2018, 140, 17666–17673. [Google Scholar] [CrossRef]
- Pathak, T.; Dey, S.; Datta, D. A metal-free, aqueous and general route to 1,5-disubstituted-1,2,3-triazoles: Reversed regioisomeric 1,3-dipolar cycloaddition of azides and vinyl sulfones. Synlett 2011, 2011, 2521–2524. [Google Scholar] [CrossRef]
- Kayet, A.; Pathak, T. 1,5-Disubstituted 1,2,3-Triazolylation at C1, C2, C3, C4, and C6 of pyranosides: A metal-free route to triazolylated monosaccharides and triazole-linked disaccharides. J. Org. Chem. 2013, 78, 9865–9875. [Google Scholar] [CrossRef]
- Kayet, A.; Dey, S.; Pathak, T. A metal free aqueous route to 1,5-disubstituted 1,2,3-triazolylated monofuranosides and difuranosides. Tetrahedron Lett. 2015, 56, 5521–5524. [Google Scholar] [CrossRef]
- Dey, S.; Pathak, T. A general route to 1,5-disubstituted 1,2,3-triazoles with alkyl/alkyl, alkyl/aryl, aryl/aryl combinations: A metal-free, regioselective, one-pot three component approach. RSC Adv. 2014, 4, 9275–9278. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Xie, Y.-Y.; Qu, H.-E.; Wang, H.-S.; Pan, Y.-M.; Huang, F.-P. Ce(OTf)3-catalyzed [3+2] cycloaddition of azides with nitroolefins: Regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles. J. Org. Chem. 2014, 79, 4463–4469. [Google Scholar] [CrossRef]
- De Nino, A.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Tallarida, M.A.; Maiuolo, L. Synthesis of 1,5-functionalized 1,2,3-triazoles using ionic liquid/Iron(III) chloride as an efficient and reusable homogeneous catalyst. Catalysts 2018, 8, 364. [Google Scholar] [CrossRef]
- Gangaprasad, D.; Raj, J.P.; Kiranmye, T.; Sasikala, R.; Karthikeyan, K.; Rani, S.K.; Elangovan, J. A tunable route to oxidative and eliminative [3+2] cycloadditions of organic azides with nitroolefins: CuO nanoparticles catalyzed synthesis of 1,2,3-triazoles under solvent-free condition. Tetrahedron Lett. 2016, 57, 3105–3108. [Google Scholar] [CrossRef]
- Kiranmye, T.; Vadivelu, M.; Sampath, S.; Muthu, K.; Karthikeyan, K. Ultrasound-assisted catalyst free synthesis of 1,4-/1,5-disubstituted-1,2,3-triazoles in aqueous medium. Sustain. Chem. Pharm. 2021, 19, 100358. [Google Scholar] [CrossRef]
- Mishra, K.B.; Tiwari, V.K. One-pot facile synthesis of 1,5-disubstituted triazolyl glycoconjugates from nitrostyrenes. Chemistryselect 2016, 1, 3693–3698. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Kumar, A.S.; Chauhan, S.; Swamy, K.C.K. Stereo- and regioselective [3+2] cycloaddition of acetoxy allenoates with azides: Metal-free synthesis of multisubstituted triazoles. Synthesis 2022, 54, 965–974. [Google Scholar] [CrossRef]
- Coats, S.J.; Link, J.S.; Gauthier, D.; Hlasta, D.J. Trimethylsilyl-directed 1,3-dipolar cycloaddition reactions in the solid-phase synthesis of 1,2,3-triazoles. Org. Lett. 2005, 7, 1469–1472. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Tang, M.; Song, X.; Chen, G.; Song, X.; Lin, Q. Potassium tert-butoxide promoted cycloaddition reaction for the synthesis of 1,5-disubstituted 1,2,3-triazoles from aromatic azides and trimethylsilyl-protected alkynes. Synlett 2012, 23, 1529–1533. [Google Scholar] [CrossRef]
- Kloss, F.; Köhn, U.; Jahn, B.O.; Hager, M.D.; Görls, H.; Schubert, U.S. Metal-free 1,5-regioselective azide-alkyne [3+2]-cycloaddition. Chem. Asian. J. 2011, 6, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Blastik, Z.E.; Klepetářová, B.; Beier, P. Enamine-mediated azide-ketone [3+2] cycloaddition of azidoperfluoroalkanes. Chemistryselect 2018, 3, 7045–7048. [Google Scholar] [CrossRef]
- González-Calderón, D.; Fuentes-Benítes, A.; Díaz-Torres, E.; González-González, C.A.; González-Romero, C. Azide-enolate 1,3-dipolar cycloaddition as an efficient approach for the synthesis of 1,5-disubstituted 1,2,3-triazoles from alkyl/aryl azides and β-ketophosphonates. Eur. J. Org. Chem. 2016, 2016, 668–672. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Tupychak, M.A.; Obushak, M.D. Metal-free synthesis of 1,5-disubstituted 1,2,3-triazoles. Russ. J. Org. Chem. 2022, 58, 209–218. [Google Scholar] [CrossRef]
- Kumar, N.; Ansari, M.Y.; Kant, R.; Kumar, A. Copper-catalyzed decarboxylative regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles. Chem. Commun. 2018, 54, 2627–2630. [Google Scholar] [CrossRef]
- Khatua, H.; Das, S.K.; Roy, S.; Chattopadhyay, B. Dual reactivity of 1,2,3,4-tetrazole: Manganese-catalyzed click reaction and denitrogenative annulation. Angew. Chem. Int. Ed. 2021, 60, 304–312. [Google Scholar] [CrossRef] [PubMed]












































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Chai, Z.; Zeng, Q.; Zhang, W.-X. Overview of 1,5-Selective Click Reaction of Azides with Alkynes or Their Synthetic Equivalents. Molecules 2023, 28, 1400. https://doi.org/10.3390/molecules28031400
Zhao Y, Chai Z, Zeng Q, Zhang W-X. Overview of 1,5-Selective Click Reaction of Azides with Alkynes or Their Synthetic Equivalents. Molecules. 2023; 28(3):1400. https://doi.org/10.3390/molecules28031400
Chicago/Turabian StyleZhao, Yaqi, Zhengqi Chai, Qingrui Zeng, and Wen-Xiong Zhang. 2023. "Overview of 1,5-Selective Click Reaction of Azides with Alkynes or Their Synthetic Equivalents" Molecules 28, no. 3: 1400. https://doi.org/10.3390/molecules28031400
APA StyleZhao, Y., Chai, Z., Zeng, Q., & Zhang, W. -X. (2023). Overview of 1,5-Selective Click Reaction of Azides with Alkynes or Their Synthetic Equivalents. Molecules, 28(3), 1400. https://doi.org/10.3390/molecules28031400