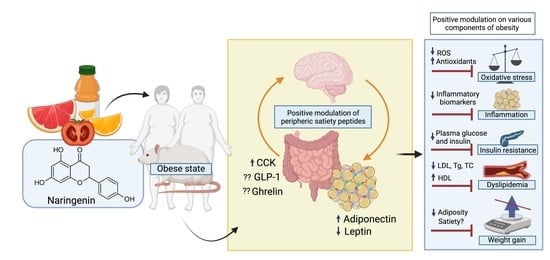
Could Naringenin Participate as a Regulator of Obesity and Satiety?
Abstract
:1. Introduction
2. General Description of Naringenin
3. Effects of Naringenin against the Various Components of Obesity
3.1. Oxidative Stress (OS)
3.2. Inflammation
3.3. Insulin Resistance
3.4. Dyslipidemia
3.5. Body Weight Control
4. The Hunger–Satiety Pathways
4.1. Ghrelin
4.2. Cholecystokinin (CCK)
4.3. Glucagon-like Peptide 1 (GLP-1)
4.4. Peptide Tyrosine-Tyrosine (PYY)
4.5. Adipokines
4.5.1. Adiponectin
4.5.2. Leptin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 27 August 2022).
- Lechner, K.; McKenzie, A.L.; Kränkel, N.; Schacky, C.V.; Worm, N.; Nixdorff, U.; Lechner, B.; Scherr, J.; Weingärtner, O.; Krauss, R.M. High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation. Metab. Syndr. Relat. Disord. 2020, 18, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.B.; Nasrullah, A.; Haq, S.; Akhtar, A.; Ghazanfar, H.; Nasir, A.; Afzal, R.M.; Bukhari, M.M.; Chaudhary, A.Y.; Naqvi, S.W. The Interplay of Genetics and Environmental Factors in the Development of Obesity. Cureus 2017, 9, e1435. [Google Scholar] [CrossRef] [PubMed]
- Boix-Castejón, M.; Herranz-López, M.; Gago, A.P.; Olivares-Vicente, M.; Caturla, N.; Roche, E.; Micol, V. Hibiscus and Lemon Verbena Polyphenols Modulate Appetite-Related Biomarkers in Overweight Subjects: A Randomized Controlled Trial. Food Funct. 2018, 9, 3173–3184. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, T.; Keszthelyi, D. Satiation or Satiety? More than Mere Semantics. Lancet 2021, 397, 1060–1061. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswami, V.; Dwoskin, L.P. Obesity: Current and Potential Pharmacotherapeutics and Targets. Pharmacol. Ther. 2017, 170, 116–147. [Google Scholar] [CrossRef] [PubMed]
- Corella-Salazar, D.A.; Domínguez-Avila, J.A.; Montiel-Herrera, M.; Astiazaran-Garcia, H.; Salazar-López, N.J.; Serafín-García, M.S.; Olivas-Orozco, G.I.; Molina-Corral, F.J.; González-Aguilar, G.A. Sub-chronic Consumption of a Phenolic-rich Avocado Paste Extract Induces GLP-1-, Leptin-, and Adiponectin-mediated Satiety in Wistar Rats. J. Food Biochem. 2021, 45, e13957. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; del Mar Contreras, M. Phenolic Compounds as Natural and Multifunctional Anti-Obesity Agents: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.-O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus Sabdariffa L. on Obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef]
- Grau-Bové, C.; González-Quilen, C.; Terra, X.; Blay, M.T.; Beltrán-Debón, R.; Jorba-Martín, R.; Espina, B.; Pinent, M.; Ardévol, A. Effects of Flavanols on Enteroendocrine Secretion. Biomolecules 2020, 10, 844. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Oluwagbemigun, K.; Schmid, M.; Nöthlings, U. Polyphenol Exposure and Risk of Type 2 Diabetes: Dose-Response Meta-Analyses and Systematic Review of Prospective Cohort Studies. Am. J. Clin. Nutr. 2018, 108, 49–61. [Google Scholar] [CrossRef]
- Sperkowska, B.; Murawska, J.; Przybylska, A.; Gackowski, M.; Kruszewski, S.; Durmowicz, M.; Rutkowska, D. Cardiovascular Effects of Chocolate and Wine—Narrative Review. Nutrients 2021, 13, 4269. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.-Y.; Kliewer, K.L.; Hamad, E.M.; Cole, R.M.; Powell, K.A.; Andridge, R.R.; Straka, S.R.; Yee, L.D.; Belury, M.A. The Flavonoid, Naringenin, Decreases Adipose Tissue Mass and Attenuates Ovariectomy-Associated Metabolic Disturbances in Mice. Nutr. Metab. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Sun, Y.; Zhang, G.; Bai, J.; Guo, J.; Su, X.; Du, H.; Cao, X.; Yang, J.; et al. Naringenin Improves Insulin Sensitivity in Gestational Diabetes Mellitus Mice through AMPK. Nutr. Diabetes 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.C.; Telford, D.E.; Edwards, J.Y.; Sutherland, B.G.; Sawyez, C.G.; Huff, M.W. Naringenin Supplementation to a Chow Diet Enhances Energy Expenditure and Fatty Acid Oxidation, and Reduces Adiposity in Lean, Pair-Fed Ldlr-/- Mice. Mol. Nutr. Food Res. 2019, 63, 1800833. [Google Scholar] [CrossRef]
- Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Morrow, M.R.; Sawyez, C.G.; Edwards, J.Y.; Drangova, M.; Huff, M.W. Intervention with Citrus Flavonoids Reverses Obesity and Improves Metabolic Syndrome and Atherosclerosis in Obese Ldlr−/− Mice. J. Lipid Res. 2018, 59, 1714–1728. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Che, J.; Yao, W. Naringenin Alleviates Obesity-Associated Hypertension by Reducing Hyperlipidemia and Oxidative Stress. Kidney Blood Press. Res. 2022, 47, 423–432. [Google Scholar] [CrossRef]
- Namkhah, Z.; Naeini, F.; Rezayat, S.M.; Yaseri, M.; Mansouri, S.; Hosseinzadeh-Attar, M.J. Does Naringenin Supplementation Improve Lipid Profile, Severity of Hepatic Steatosis and Probability of Liver Fibrosis in Overweight/Obese Patients with NAFLD? A Randomised, Double-blind, Placebo-controlled, Clinical Trial. Int. J. Clin. Pract. 2021, 75, e14852. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, Pharmacodynamic and Formulations Aspects of Naringenin: An Update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and Pharmacokinetics of Naringenin: A Randomized, Controlled, Single-ascending-dose Clinical Trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
- De Lourdes Mata-Bilbao, M.; Andrés-Lacueva, C.; Roura, E.; Jáuregui, O.; Escribano, E.; Torre, C.; Lamuela-Raventós, R.M. Absorption and Pharmacokinetics of Grapefruit Flavanones in Beagles. Br. J. Nutr. 2007, 98, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Kulkarni, K.H.; Singh, R.; Yang, Z.; Wang, S.W.J.; Tam, V.H.; Hu, M. Disposition of Naringenin via Glucuronidation Pathway Is Affected by Compensating Efflux Transporters of Hydrophilic Glucuronides. Mol. Pharm. 2009, 6, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Chabane, M.N.; Ahmad, A.A.; Peluso, J.; Muller, C.D.; Ubeaud, G. Quercetin and Naringenin Transport across Human Intestinal Caco-2 Cells. J. Pharm. Pharmacol. 2009, 61, 1473–1483. [Google Scholar] [CrossRef]
- Zhang, Z.-D.; Tao, Q.; Qin, Z.; Liu, X.-W.; Li, S.-H.; Bai, L.-X.; Yang, Y.-J.; Li, J.-Y. Uptake and Transport of Naringenin and Its Antioxidant Effects in Human Intestinal Epithelial Caco-2 Cells. Front. Nutr. 2022, 9, 894117. [Google Scholar] [CrossRef]
- Zeng, X.; Yao, H.; Zheng, Y.; He, Y.; He, Y.; Rao, H.; Li, P.; Su, W. Tissue Distribution of Naringin and Derived Metabolites in Rats after a Single Oral Administration. J. Chromatogr. B 2020, 1136, 121846. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.-H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxidative Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef] [Green Version]
- Ávila-Escalante, M.L.; Coop-Gamas, F.; Cervantes-Rodríguez, M.; Méndez-Iturbide, D.; Aranda-González, I.I. The Effect of Diet on Oxidative Stress and Metabolic Diseases-Clinically Controlled Trials. J. Food Biochem. 2020, 44, e13191. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, R.; Magesh, S.B.; Ramkumar, K.M.; Suryanarayanan, S.; SubbaRao, M.V. Antioxidant Potential of Naringenin Helps to Protect Liver Tissue from Streptozotocin-Induced Damage. Rep. Biochem. Mol. Biol. 2018, 7, 76–84. [Google Scholar]
- Veiko, A.G.; Lapshina, E.A.; Zavodnik, I.B. Comparative Analysis of Molecular Properties and Reactions with Oxidants for Quercetin, Catechin, and Naringenin. Mol. Cell. Biochem. 2021, 476, 4287–4299. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial Effects of Naringenin in Liver Diseases: Molecular Mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Influence of Membrane Lipid Composition on Flavonoid–Membrane Interactions: Implications on Their Biological Activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Veiko, A.G.; Sekowski, S.; Lapshina, E.A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zamaraeva, M.; Zhao, H.; Zavodnik, I.B. Flavonoids Modulate Liposomal Membrane Structure, Regulate Mitochondrial Membrane Permeability and Prevent Erythrocyte Oxidative Damage. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2020, 1862, 183442. [Google Scholar] [CrossRef]
- Xu, S.; Wu, B.; Zhong, B.; Lin, L.; Ding, Y.; Jin, X.; Huang, Z.; Lin, M.; Wu, H.; Xu, D. Naringenin Alleviates Myocardial Ischemia/Reperfusion Injury by Regulating the Nuclear Factor-Erythroid Factor 2-Related Factor 2 (Nrf2)/System Xc-/Glutathione Peroxidase 4 (GPX4) Axis to Inhibit Ferroptosis. Bioengineered 2021, 12, 10924–10934. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ye, Y.; Wu, Z.; Lin, J.; Wang, Y.; Ding, Q.; Yang, X.; Yang, W.; Lin, B.; Lin, B. Temporary Upregulation of Nrf2 by Naringenin Alleviates Oxidative Damage in the Retina and ARPE-19 Cells. Oxidative Med. Cell. Longev. 2021, 2021, 4053276. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxidative Med. Cell. Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H. Naringenin Inhibit the Hydrogen Peroxide-Induced SH-SY5Y Cells Injury through Nrf2/HO-1 Pathway. Neurotox. Res. 2019, 36, 796–805. [Google Scholar] [CrossRef]
- Hua, Y.Q.; Zeng, Y.; Xu, J.; Xu, X.L. Naringenin Alleviates Nonalcoholic Steatohepatitis in Middle-Aged Apoe−/−mice: Role of SIRT1. Phytomedicine 2021, 81, 153412. [Google Scholar] [CrossRef] [PubMed]
- Manchope, M.F.; Artero, N.A.; Fattori, V.; Mizokami, S.S.; Pitol, D.L.; Issa, J.P.M.; Fukada, S.Y.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; et al. Naringenin Mitigates Titanium Dioxide (TiO2)-Induced Chronic Arthritis in Mice: Role of Oxidative Stress, Cytokines, and NFκB. Inflamm. Res. 2018, 67, 997–1012. [Google Scholar] [CrossRef]
- Wali, A.F.; Rashid, S.; Rashid, S.M.; Ansari, M.A.; Khan, M.R.; Haq, N.; Alhareth, D.Y.; Ahmad, A.; Rehman, M.U. Naringenin Regulates Doxorubicin-Induced Liver Dysfunction: Impact on Oxidative Stress and Inflammation. Plants 2020, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Ngo, C.; Willcox, J.C.; Lappas, M. Anti-Diabetic, Anti-Inflammatory, and Anti-Oxidant Effects of Naringenin in an In Vitro Human Model and an In Vivo Murine Model of Gestational Diabetes Mellitus. Mol. Nutr. Food Res. 2019, 63, 1900224. [Google Scholar] [CrossRef]
- Kometsi, L.; Govender, K.; Mato, E.P.M.; Hurchund, R.; Owira, P.M.O. By Reducing Oxidative Stress, Naringenin Mitigates Hyperglycaemia-Induced Upregulation of Hepatic Nuclear Factor Erythroid 2-Related Factor 2 Protein. J. Pharm. Pharmacol. 2020, 72, 1394–1404. [Google Scholar] [CrossRef]
- Baranowska, M.; Koziara, Z.; Suliborska, K.; Chrzanowski, W.; Wormstone, M.; Namieśnik, J.; Bartoszek, A. Interactions between Polyphenolic Antioxidants Quercetin and Naringenin Dictate the Distinctive Redox-Related Chemical and Biological Behaviour of Their Mixtures. Sci. Rep. 2021, 11, 12282. [Google Scholar] [CrossRef]
- Fernø, J.; Strand, K.; Mellgren, G.; Stiglund, N.; Björkström, N.K. Natural Killer Cells as Sensors of Adipose Tissue Stress. Trends Endocrinol. Metab. 2020, 31, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. -Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Tsuhako, R.; Yoshida, H.; Sugita, C.; Kurokawa, M. Naringenin Suppresses Neutrophil Infiltration into Adipose Tissue in High-Fat Diet-Induced Obese Mice. J. Nat. Med. 2020, 74, 229–237. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, H.; Ishida, A.; Watanabe, W.; Narumi, K.; Atsumi, T.; Sugita, C.; Kurokawa, M. Naringenin Suppresses Macrophage Infiltration into Adipose Tissue in an Early Phase of High-Fat Diet-Induced Obesity. Biochem. Biophys. Res. Commun. 2014, 454, 95–101. [Google Scholar] [CrossRef]
- Beddows, C.A.; Dodd, G.T. Insulin on the Brain: The Role of Central Insulin Signalling in Energy and Glucose Homeostasis. J. Neuroendocrinol. 2021, 33, e12947. [Google Scholar] [CrossRef] [PubMed]
- Hallschmid, M.; Higgs, S.; Thienel, M.; Ott, V.; Lehnert, H. Postprandial Administration of Intranasal Insulin Intensifies Satiety and Reduces Intake of Palatable Snacks in Women. Diabetes 2012, 61, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, K.T.; Subhedar, N.K.; Kokare, D.M. Involvement of Neuropeptide CART in the Central Effects of Insulin on Feeding and Body Weight. Pharmacol. Biochem. Behav. 2019, 181, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.K.; Veeraraghavan, V.P.; Palanisamy, C.P.; Cui, B.; Periyasamy, V.; Chandrasekar, K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-ΚB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules 2021, 26, 2101. [Google Scholar] [CrossRef]
- Bu, L.; Cao, X.; Zhang, Z.; Wu, H.; Guo, R.; Ma, M. Decreased Secretion of Tumor Necrosis Factor-α Attenuates Macrophages-Induced Insulin Resistance in Skeletal Muscle. Life Sci. 2020, 244, 117304. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Oksbjerg, N.; Young, J.F.; Jeppesen, P.B. Caffeic Acid, Naringenin and Quercetin Enhance Glucose-Stimulated Insulin Secretion and Glucose Sensitivity in INS-1E Cells. Diabetes Obes. Metab. 2014, 16, 602–612. [Google Scholar] [CrossRef]
- Jia, B.; Wang, Y.; Yu, G.; Cheng, Y.; Yang, C.; Cao, F.; He, Y.; Cao, P.; Meng, X.; Yu, D. Naringenin Ameliorates Insulin Resistance by Modulating Endoplasmic Reticulum Stress in Hepatitis C Virus-Infected Liver. Biomed. Pharmacother. 2019, 115, 108848. [Google Scholar] [CrossRef]
- Singh, A.K.; Raj, V.; Keshari, A.K.; Rai, A.; Kumar, P.; Rawat, A.; Maity, B.; Kumar, D.; Prakash, A.; De, A.; et al. Isolated Mangiferin and Naringenin Exert Antidiabetic Effect via PPAR γ/GLUT4 Dual Agonistic Action with Strong Metabolic Regulation. Chem.-Biol. Interact. 2018, 280, 33–44. [Google Scholar] [CrossRef]
- Costa-Urrutia, P.; Colistro, V.; Franco-Trecu, V.; Granados, J.; Fariña, R.Á.; Rodríguez-Arellano, M.E. Dyslipidemia, Obesity, and Ethnicity in Mexican Children. Int. J. Environ. Res. Public Health 2021, 18, 12659. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and Dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines Formedical Care of Patients with Obesity. Endocr. Pract. 2016, 22, 1–203. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Greenway, F.L.; Lau, F.H.; Lin, Y.; Stephens, J.M.; Johnson, W.D.; Coulter, A.A. Naringenin Promotes Thermogenic Gene Expression in Human White Adipose Tissue. Obesity 2019, 27, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Zou, J.; Wang, Y.-H.; Xu, M.-X.; Huang, W.; Yu, D.-J.; Zhang, L.; Zhang, Y.-Y.; Sun, X.-D. Naringenin Attenuates Non-Alcoholic Fatty Liver Disease by Enhancing Energy Expenditure and Regulating Autophagy via AMPK. Front. Pharmacol. 2021, 12, 687095. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Schutz, D.D.; Busetto, L.; Dicker, D.; Farpour-Lambert, N.; Pryke, R.; Toplak, H.; Widmer, D.; Yumuk, V.; Schutz, Y. European Practical and Patient-Centred Guidelines for Adult Obesity Management in Primary Care. Obes. Facts 2019, 12, 40–66. [Google Scholar] [CrossRef]
- Wharton, S.; Lau, D.C.W.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in Adults: A Clinical Practice Guideline. Can. Med. Assoc. J. 2020, 192, E875–E891. [Google Scholar] [CrossRef]
- Melhem, S.; Steven, S.; Taylor, R.; Al-Mrabeh, A. Effect of Weight Loss by Low-Calorie Diet on Cardiovascular Health in Type 2 Diabetes: An Interventional Cohort Study. Nutrients 2021, 13, 1465. [Google Scholar] [CrossRef]
- Yoshino, M.; Kayser, B.D.; Yoshino, J.; Stein, R.I.; Reeds, D.; Eagon, J.C.; Eckhouse, S.R.; Watrous, J.D.; Jain, M.; Knight, R.; et al. Effects of Diet versus Gastric Bypass on Metabolic Function in Diabetes. N. Engl. J. Med. 2020, 383, 721–732. [Google Scholar] [CrossRef]
- Murugesan, N.; Woodard, K.; Ramaraju, R.; Greenway, F.L.; Coulter, A.A.; Rebello, C.J. Naringenin Increases Insulin Sensitivity and Metabolic Rate: A Case Study. J. Med. Food 2020, 23, 343–348. [Google Scholar] [CrossRef]
- Ortiz-Andrade, R.; Araujo León, J.A.; Sánchez-Salgado, J.C.; Sánchez-Recillas, A.; Vazquez-Garcia, P.; Hernández-Núñez, E. Citroflavonoids as Promising Agents for Drug Discovery in Diabetes and Hypertension: A Systematic Review of Experimental Studies. Molecules 2022, 27, 7933. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.; Sanz-Lamora, H.; Arias, G.; Marrero, P.F.; Haro, D.; Relat, J. Metabolic Impact of Flavonoids Consumption in Obesity: From Central to Peripheral. Nutrients 2020, 12, 2393. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent For Obesity. DDDT 2019, 13, 3855–3866. [Google Scholar] [CrossRef]
- Fukuchi, Y.; Hiramitsu, M.; Okada, M.; Hayashi, S.; Nabeno, Y.; Osawa, T.; Naito, M. Lemon Polyphenols Suppress Diet-Induced Obesity by Up-Regulation of MRNA Levels of the Enzymes Involved in Beta-Oxidation in Mouse White Adipose Tissue. J. Clin. Biochem. Nutr. 2008, 43, 201–209. [Google Scholar] [CrossRef]
- Bellisle, F.; Drewnowski, A.; Anderson, G.H.; Westerterp-Plantenga, M.; Martin, C.K. Sweetness, Satiation, and Satiety. J. Nutr. 2012, 142, 1149S–1154S. [Google Scholar] [CrossRef]
- Clemmensen, C.; Müller, T.D.; Woods, S.C.; Berthoud, H.-R.; Seeley, R.J.; Tschöp, M.H. Gut-Brain Cross-Talk in Metabolic Control. Cell 2017, 168, 758–774. [Google Scholar] [CrossRef]
- Page, A.J.; Christie, S.; Symonds, E.; Li, H. Circadian Regulation of Appetite and Time Restricted Feeding. Physiol. Behav. 2020, 220, 112873. [Google Scholar] [CrossRef]
- Yasrebi, A.; Hsieh, A.; Mamounis, K.J.; Krumm, E.A.; Yang, J.A.; Magby, J.; Hu, P.; Roepke, T.A. Differential Gene Regulation of GHSR Signaling Pathway in the Arcuate Nucleus and NPY Neurons by Fasting, Diet-Induced Obesity, and 17β-Estradiol. Mol. Cell. Endocrinol. 2016, 422, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.M.I. Central and Peripheral Control of Food Intake. Endocr. Regul. 2017, 51, 52–70. [Google Scholar] [CrossRef] [PubMed]
- De Ceglia, M.; Decara, J.; Gaetani, S.; de Fonseca, F.R. Obesity as a Condition Determined by Food Addiction: Should Brain Endocannabinoid System Alterations Be the Cause and Its Modulation the Solution? Pharmaceuticals 2021, 14, 1002. [Google Scholar] [CrossRef]
- Sudakov, S.; Bogdanova, N. Involvement of Peripheral Opioid Receptors in the Realization of Food Motivation into Eating Behavior. Front. Behav. Neurosci. 2021, 14, 600920. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Q.; Zhou, Q.; Chen, Y.; Lei, X.; Chen, Y.; Chen, Q. Circulating Acyl and Des-Acyl Ghrelin Levels in Obese Adults: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 2679. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, T.-K.; Shim, W.-S. Naringin Exhibits in Vivo Prokinetic Activity via Activation of Ghrelin Receptor in Gastrointestinal Motility Dysfunction Rats. Pharmacology 2013, 92, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Karavetian, M.; Moubareck, C.A.; Wazz, G.; Mahdy, T.; Venema, K. The Association between Peptide Hormones with Obesity and Insulin Resistance Markers in Lean and Obese Individuals in the United Arab Emirates. Nutrients 2022, 14, 1271. [Google Scholar] [CrossRef] [PubMed]
- Mesgari-Abbasi, M. Serum Concentrations of Cholecystokinin, Peptide YY, Ghrelin and High Sensitive C-Reactive Protein in Association with Metabolic Syndrome Ingredients in Obese Individuals. Acta Endocrinol. 2020, 16, 37–42. [Google Scholar] [CrossRef]
- Park, M.; Kim, K.; Lee, Y.M.; Rhyu, M.R.; Kim, H.Y. Naringenin Stimulates Cholecystokinin Secretion in STC-1 Cells. Nutr. Res. Pract. 2014, 8, 146–150. [Google Scholar] [CrossRef]
- Krieger, J.-P. Intestinal Glucagon-like Peptide-1 Effects on Food Intake: Physiological Relevance and Emerging Mechanisms. Peptides 2020, 131, 170342. [Google Scholar] [CrossRef]
- Lampropoulos, C.; Alexandrides, T.; Tsochatzis, S.; Kehagias, D.; Kehagias, I. Are the Changes in Gastrointestinal Hormone Secretion Necessary for the Success of Bariatric Surgery? A Critical Review of the Literature. Obes. Surg. 2021, 31, 4575–4584. [Google Scholar] [CrossRef]
- Andreoli, M.F.; Donato, J.; Cakir, I.; Perello, M. Leptin Resensitisation: A Reversion of Leptin-Resistant States. J. Endocrinol. 2019, 241, R81–R96. [Google Scholar] [CrossRef]
- Engin, A. Adiponectin-Resistance in Obesity. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 960, pp. 415–441. ISBN 978-3-319-48380-1. [Google Scholar]
- Ahmed, O.M.; Hassan, M.A.; Abdel-Twab, S.M.; Azeem, M.N.A. Navel Orange Peel Hydroethanolic Extract, Naringin and Naringenin Have Anti-Diabetic Potentials in Type 2 Diabetic Rats. Biomed. Pharmacother. 2017, 94, 197–205. [Google Scholar] [CrossRef]
- Smith, K.R.; Moran, T.H. Gastrointestinal Peptides in Eating-Related Disorders. Physiol. Behav. 2021, 238, 113456. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating Ghrelin Levels Are Decreased in Human Obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Witjaksono, F.; Simadibrata, M.; Lukito, W.; Wijaya, A.; Nurwidya, F. Profiles of Peptide YY and Ghrelin, Levels of Hunger and Satiety, and Ad Libitum Intake in Obese and Non-Obese Indonesian Women. Rom. J. Intern. Med. 2019, 57, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.I.; Enriori, P.J.; Lemus, M.B.; Cowley, M.A.; Andrews, Z.B. Diet-Induced Obesity Causes Ghrelin Resistance in Arcuate NPY/AgRP Neurons. Endocrinology 2010, 151, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Zigman, J.M.; Bouret, S.G.; Andrews, Z.B. Obesity Impairs the Action of the Neuroendocrine Ghrelin System. Trends Endocrinol. Metab. TEM 2016, 27, 54–63. [Google Scholar] [CrossRef]
- Montalbano, G.; Maugeri, A.; Guerrera, M.C.; Miceli, N.; Navarra, M.; Barreca, D.; Cirmi, S.; Germanà, A. A White Grape Juice Extract Reduces Fat Accumulation through the Modulation of Ghrelin and Leptin Expression in an In Vivo Model of Overfed Zebrafish. Molecules 2021, 26, 1119. [Google Scholar] [CrossRef]
- Pathak, V.; Flatt, P.R.; Irwin, N. Cholecystokinin (CCK) and Related Adjunct Peptide Therapies for the Treatment of Obesity and Type 2 Diabetes. Peptides 2018, 100, 229–235. [Google Scholar] [CrossRef]
- Mhalhal, T.R.; Washington, M.C.; Newman, K.; Heath, J.C.; Sayegh, A.I. Exogenous Glucagon-like Peptide-1 Reduces Body Weight and Cholecystokinin-8 Enhances This Reduction in Diet-Induced Obese Male Rats. Physiol. Behav. 2017, 179, 191–199. [Google Scholar] [CrossRef]
- Cawthon, C.R.; de La Serre, C.B. The Critical Role of CCK in the Regulation of Food Intake and Diet-Induced Obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef]
- Feng, B.; Harms, J.; Patel, N.; Ye, H.; Luo, P.; Irizarry, V.T.; Vidrine, J.; Coulter, A.; Rebello, C.J.; Yu, S.; et al. Targeting the T-Type Calcium Channel Cav3.2 in GABAergic Arcuate Nucleus Neurons to Treat Obesity. Mol. Metab. 2021, 54, 101391. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, M.; Kim, K.; Lee, Y.M.; Rhyu, M.R. Hesperetin Stimulates Cholecystokinin Secretion in Enteroendocrine STC-1 Cells. Biomol. Ther. 2013, 21, 121–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukor, N.A.; Ravallec, R.; Camp, J.V.; Raes, K.; Smagghe, G. Flavonoids Stimulate Cholecystokinin Peptide Secretion from the Enteroendocrine STC-1 Cells. Fitoterapia 2016, 113, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Shukor, N.A.; Raes, K.; Camp, J.V.; Smagghe, G. Analysis of Interaction of Phenolic Compounds with the Cholecystokinin Signaling Pathway to Explain Effects on Reducing Food Intake. Peptides 2014, 53, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3–36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, X. Computational Investigation of Flavonol-Based GLP-1R Agonists Using DFT Calculations and Molecular Docking. Comput. Theor. Chem. 2020, 1190, 113005. [Google Scholar] [CrossRef]
- Grill, H.J. A Role for GLP-1 in Treating Hyperphagia and Obesity. Endocrinology 2020, 161, bqaa093. [Google Scholar] [CrossRef]
- Lee, L.-C.; Hou, Y.-C.; Hsieh, Y.-Y.; Chen, Y.-H.; Shen, Y.-C.; Lee, I.-J.; Shih, M.-C.M.; Hou, W.-C.; Liu, H.-K. Dietary Supplementation of Rutin and Rutin-Rich Buckwheat Elevates Endogenous Glucagon-like Peptide 1 Levels to Facilitate Glycemic Control in Type 2 Diabetic Mice. J. Funct. Foods 2021, 85, 104653. [Google Scholar] [CrossRef]
- Liu, L.; Sayama, K. The Combined Administration of EGCG and Caffeine Induces Not Only Suppression of Fat Accumulation but Also Anorexigenic Action in Mice. J. Funct. Foods 2018, 47, 156–162. [Google Scholar] [CrossRef]
- Obaroakpo, J.U.; Liu, L.; Zhang, S.; Lu, J.; Liu, L.; Pang, X.; Lv, J. In Vitro Modulation of Glucagon-like Peptide Release by DPP-IV Inhibitory Polyphenol-Polysaccharide Conjugates of Sprouted Quinoa Yoghurt. Food Chem. 2020, 324, 126857. [Google Scholar] [CrossRef]
- Rehman, K.; Ali, M.B.; Akash, M.S.H. Genistein Enhances the Secretion of Glucagon-like Peptide-1 (GLP-1) via Downregulation of Inflammatory Responses. Biomed. Pharmacother. 2019, 112, 108670. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.; Alkhalidy, H.; Luo, J.; Moomaw, E.; Neilson, A.P.; Liu, D. Flavone Hispidulin Stimulates Glucagon-Like Peptide-1 Secretion and Ameliorates Hyperglycemia in Streptozotocin-Induced Diabetic Mice. Mol. Nutr. Food Res. 2020, 64, 1900978. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yao, D.; Yang, H.; Wei, Y.; Peng, Y.; Ding, Y.; Shu, L. Puerarin Protects Pancreatic β-Cells in Obese Diabetic Mice via Activation of GLP-1R Signaling. Mol. Endocrinol. 2016, 30, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, M.; Salinas, C.B.G.; Rehfeld, J.F.; Secher, A.; Raun, K.; Wulff, B.S. PYY(3-36) and Exendin-4 Reduce Food Intake and Activate Neuronal Circuits in a Synergistic Manner in Mice. Neuropeptides 2019, 73, 89–95. [Google Scholar] [CrossRef]
- Huang, H.-H.; Wang, T.-Y.; Yao, S.-F.; Lin, P.-Y.; Chang, J.C.-Y.; Peng, L.-N.; Chen, L.-K.; Yen, D.H.-T. Gastric Mobility and Gastrointestinal Hormones in Older Patients with Sarcopenia. Nutrients 2022, 14, 1897. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-Y.; Aihara, Y.; Hashimoto, T.; Kanazawa, K.; Mizuno, M. (−)-Epigallocatechin-3-Gallate Induces Secretion of Anorexigenic Gut Hormones. J. Clin. Biochem. Nutr. 2015, 57, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, Y.; Yao, T.; Huang, Y.; He, Z.; Kong, X.; Yu, K.; Wang, R.; Guo, H.; Yan, J.; et al. Adiponectin Potentiates the Acute Effects of Leptin in Arcuate Pomc Neurons. Mol. Metab. 2016, 5, 882–891. [Google Scholar] [CrossRef]
- Galley, J.C.; Singh, S.; Awata, W.M.C.; Alves, J.V.; Bruder-Nascimento, T. Adipokines: Deciphering the Cardiovascular Signature of Adipose Tissue. Biochem. Pharmacol. 2022, 206, 115324. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kim, J.-S.; Jo, M.-J.; Cho, E.; Ahn, S.-Y.; Kwon, Y.-J.; Ko, G.-J. The Roles and Associated Mechanisms of Adipokines in Development of Metabolic Syndrome. Molecules 2022, 27, 334. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Dludla, P.V.; Mabasa, L.; Kappo, A.P.; Basson, A.K.; Pheiffer, C.; Johnson, R. The Effect of Adiponectin in the Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD) and the Potential Role of Polyphenols in the Modulation of Adiponectin Signaling. Biomed. Pharmacother. 2020, 131, 110785. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Vatner, D.F.; Goedeke, L.; Hirabara, S.M.; Zhang, Y.; Perry, R.J.; Shulman, G.I. Mechanisms by Which Adiponectin Reverses High Fat Diet-Induced Insulin Resistance in Mice. Proc. Natl. Acad. Sci. USA 2020, 117, 32584–32593. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Pu, H.; Wei, Q.; Duan, M.; Zhang, C.; Jiang, T.; Shou, X.; Zhang, J.; Yang, Y. Adiponectin Improves NF-ΚB-Mediated Inflammation and Abates Atherosclerosis Progression in Apolipoprotein E-Deficient Mice. Lipids Health Dis. 2016, 15, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin Stimulates Glucose Utilization and Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shan, S.; Zhang, K.; Ning, Z.-Q.; Lu, X.-P.; Cheng, Y.-Y. Naringenin and Hesperetin, Two Flavonoids Derived from Citrus Aurantium up-Regulate Transcription of Adiponectin. Phytother. Res. 2008, 22, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Horiba, T.; Nishimura, I.; Nakai, Y.; Abe, K.; Sato, R. Naringenin Chalcone Improves Adipocyte Functions by Enhancing Adiponectin Production. Mol. Cell. Endocrinol. 2010, 323, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Vega, J.L.; Raffoul-Orozco, A.K.; Hernandez-Molina, D.; Ávila-González, A.E.; García-Cobian, T.A.; Rubio-Arellano, E.D.; Ramirez-Lizardo, E.J. Naringin Reduces Body Weight, Plasma Lipids and Increases Adiponectin Levels in Patients with Dyslipidemia. Int. J. Vitam. Nutr. Res. 2022, 92, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wagner, E.J.; Rønnekleiv, O.K.; Kelly, M.J. Insulin and Leptin Excite Anorexigenic Pro-Opiomelanocortin Neurones via Activation of TRPC5 Channels. J. Neuroendocrinol. 2018, 30, e12501. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.T.; Decherf, S.; Loh, K.; Simonds, S.E.; Wiede, F.; Balland, E.; Merry, T.L.; Münzberg, H.; Zhang, Z.-Y.; Kahn, B.B.; et al. Leptin and Insulin Act on POMC Neurons to Promote the Browning of White Fat. Cell 2015, 160, 88–104. [Google Scholar] [CrossRef]
- Tanida, M.; Iwasaki, Y.; Yamamoto, N. Central Injection of Leptin Increases Sympathetic Nerve Outflows to the Stomach and Spleen in Anesthetized Rats. In Vivo 2019, 33, 1827–1832. [Google Scholar] [CrossRef]
- Koizumi, H.; Mohammad, S.; Ozaki, T.; Muto, K.; Matsuba, N.; Kim, J.; Pan, W.; Morioka, E.; Mochizuki, T.; Ikeda, M. Intracellular Interplay between Cholecystokinin and Leptin Signalling for Satiety Control in Rats. Sci. Rep. 2020, 10, 12000. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, T.; Li, C.; Yang, G. STAT3 Phosphorylation in Central Leptin Resistance. Nutr. Metab. 2021, 18, 39. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Chhoker, S.S.; Sawyez, C.G.; Drangova, M.; Adams, A.C.; Kharitonenkov, A.; et al. Naringenin Prevents Obesity, Hepatic Steatosis, and Glucose Intolerance in Male Mice Independent of Fibroblast Growth Factor 21. Endocrinology 2015, 156, 2087–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Experimental Model (Reference) | Dose and Route of Administration | Time | Effects | Findings |
|---|---|---|---|---|
| C57BL/6J obese male mice, HFD (60% calories from fats) [50] | 100 mg/kg/day, P.O. | 14 days | Antiinflammatory | NAR suppresses neutrophil infiltration in adipose tissue secondary to an HFD (p < 0.05 for HFD vs. HFD + NAR). Decreasing trend in the expression of MCP-1, IL-6, MIP-1α, MIP-2 and significant decrease in MCP-3 in adipose tissue (p < 0.05 for HFD vs. HFD + NAR). |
| Female mice with gestational diabetes, heterozygotes B6.BKS(D)-Lepr ᵈᵇ/⁺/J [45] | 50 mg/kg dissolved in dimethyl sulfoxide (DMSO), I.P. | 8 days | Antihyperglycemic | NAR lowers fasting glycemia by 15% (p = 0.0127 vs. control). |
| Antiinflammatory | Significant decrease in IL-1A mRNA expression in visceral adipose tissue (p < 0.05 vs. control). | |||
| Antioxidant | NAR increased GR mRNA expression (p < 0.05 vs. control), as well as decreased CAT mRNA expression (p < 0.05 vs. control) in visceral adipose tissue. NAR increased mRNA expression of SOD1 (p < 0.05 vs. control) in subcutaneous adipose tissue. | |||
| C57BLKsJ db/+ (db/+) mice. Standard diet (29% protein, 47% carbohydrates, 17% fats) [16] | 100 mg/kg/bw/day Oral gavage, 1% CMC | 4 weeks | Antihyperglycemic | NAR lowered glycemia (0 min p < 0.05 GDM vs. GDM + NAR; 30 min p < 0.05 GDM vs. GDM + NAR; 60 min p < 0.05 GDM vs. GDM + NAR; 90 min p < 0.01 GDM vs. GDM + NAR; 120 min p < 0.01 GDM vs. GDM + NAR) (fasting glycemia 6.84 ± 1.03 mmol/L GDM vs. 4.38 ± 0.89 mmol/L GDM + NAR, p < 0.05), improved glycemic profile, including HOMA-IR (9.52 ± 0.31 GDM vs. 6.12 ± 0.23 GDM + NAR, p < 0.05), but without normalizing to the parental strain. |
| Antiinflammatory | Significant decrease in proinflammatory cytokines in serum and skeletal muscle (GDM vs. GDM + NAR), IL-1β (p < 0.05 serum, p < 0.01 skeletal m.), IL-6 (p < 0.01 serum and skeletal m.), TNF-α (p < 0.01 serum and skeletal m.) and MCP-1 (p < 0.01 serum, p < 0.05 skeletal m.). | |||
| Antiobesogenic | Significantly lower body weight gain, without weight normalization when compared to the parental strain (p < 0.05 for GDM vs. GDM + NAR). | |||
| In vitro, C2C12 mouse myoblasts [16] | 50 μg/mL NAR | - | Antihyperglycemic | Increased AMPK-dependent membrane translocation of GLUT4. |
| Antioxidant | NAR decreases ROS levels in C2C12 cells treated with TNF-α, in an AMPK-dependent manner. | |||
| In vivo, male LDLR−/− mice. Rodent chow (12% of calories from fat, 16% protein; isocaloric diet) [17] | NAR in diet 3% wt/wt (supplementation). P.O. | 8 weeks | Antihyperglycemic | NAR decreased fasting glycemia by 37% (p < 0.05 for chow vs. chow + NAR), fasting insulinemia by 57% (p < 0.05 for chow vs. chow + NAR) and improved HOMA-IR (p < 0.05 for chow vs. chow + NAR). |
| Antihyperlipidemic | Decreased levels of TG and TC (46% and 23%, respectively; p < 0.05 for chow vs. chow + NAR). Increased fatty acid oxidation in the liver through increased serum levels of ꞵ-hydroxybutyrate (33%; p < 0.05 for chow vs. chow + NAR) and increased expression of hepatic genes involved in fatty acid oxidation (PGC-1a, 47%, p < 0.05 for chow vs. chow + NAR; Cpt1a, 15%, trend) and lipolysis (Pnpla2(ATGL) 33%, p < 0.05 for chow vs. chow + NAR). | |||
| Antiobesogenic Satiety | Reduced in weight gain (~8–10%; p < 0.05 for chow vs. chow + NAR) by decreased adiposity (eWAT (69%) and iWAT (71%), (p < 0.05 for chow vs. chow +NAR) and increased energy expenditure. No significant effects on satiety. | |||
| Antihyperglycemic | Reversed insulinemia by 50%. Decreased fasting glycemia (trend-13%). Improved HOMA-IR. | |||
| Obese LDLR−/− male mice. High fat and cholesterol diet (HFHC) (42% of calories from fat, 0.2% cholesterol) [18] | NAR 3% wt/wt dietary supplementation. P.O. | 12 weeks | Antiinflammatory | Decreased mRNA expression of TNF-α, Ccl2 and Ccl3 (trend). |
| Antihyperlipidemic | Reduced total cholesterol (TC) and TG by ˃50% (p < 0.05 HFHC vs. HFHC + NAR) | |||
| Antiobesogenic | Decreased adipose tissue hypertrophy with decreased epididymal adipocyte area by 19% (p < 0.05 HFHC vs. HFHC + NAR) and epididymal tissue reduction by 29% (p < 0.05 HFHC vs. HFHC + NAR). Induced body weight loss of ~13% after intervention (p < 0.05). | |||
| Hepatoprotective | Reversed intrahepatic TG at the end of the experimental period (58–82%) (p < 0.05 HFHC vs. HFHC + NAR). Reversed suppression of genes involved in β-oxidation, increasing its expression up to 1.4× (Cpt1α) and reduces (trend) Srebp1c, suggesting reduction in de novo lipogenesis. | |||
| Satiety | Intervention with NAR + HFHC showed no significant changes in food intake between groups. An aversion to NAR taste initially documented, which decreased slowly increasing the dose of the flavonoid in the first week to prevent a significant impact on food intake. | |||
| Antihyperglycemic | Decreased fasting glycemia at week 18 (163.0 ± 5.2 mg/dL vs. 127.1 ± 10.2 mg/dL; p < 0.05 CT vs. NAR) and HOMA-IR (p < 0.05 CT vs. NAR). | |||
| In vivo, intervention model. Ovariectomized female C57BL/6J mice. Semi-purified diet (control) protein 20% kcal, carbohydrates 70%, fats 10% [15] | NAR 3% wt/wt | 11 weeks (after 11 weeks of induction) | Antiinflammatory | Decreased mRNA expression of MCP1 (56%) and IL-6 (40%) in perigonadal adipose tissue (p < 0.05 CT vs. NAR). |
| Antihyperlipidemic | Decreased serum TC (p < 0.05 CT vs. NAR), evident by H&E staining. Increased mRNA expression of Srebp1, Cpt1α, PGC1α (4-fold and PEPCK (3.5-fold) (p < 0.05 CT vs. NAR). | |||
| Hepatoprotective | Decreased total lipids and TG in the liver (p < 0.05 CT vs. NAR) | |||
| Antiobesogenic | Decreased body weight (p < 0.05 CT vs. NAR). Decrease in total adiposity (intra-abdominal and subcutaneous) of 54, 59 and 50%, respectively (p < 0.05 CT vs. NAR). | |||
| Satiety | Decreased leptin by 80% (p < 0.05 CT vs. NAR). Decreased caloric intake (~14%; week 12, p < 0.05 CT vs. NAR; week 13–22, trend, p = 0.075). | |||
| Antilipidemic | Increased gene expression associated with thermogenesis and fat oxidation: UCP1, PGC-1α and PGC-1β, ATGL, CPT1β (p < 0.01, CT vs. NAR). | |||
| In vitro, human white adipocyte culture (hADSC) and abdominal subcutaneous white adipose tissue (pWAT) from human subjects. [64] | 8 μM | 7 days for hADSC | Antihyperglycemic | Increased mRNA expression of GLUT4, ChREBP α + β, adiponectin (p < 0.01, CT vs. NAR). |
| Antidyslipidemic | Significantly decreases TG, TC and LDL (p < 0.05, HFD vs. NAR; 50 and 100 mg/kg) Increases HDL-c (p < 0.05, HFD vs. NAR; 50 and 100 mg/kg) | |||
| Male Wistar rats. High-fat diet (22% protein, 27% fats, 41% carbohydrates) [19] | NAR 25,50 and 100 mg/kg, dissolved in DMSO | 4 weeks | Antioxidant | Significant decrease in plasma MDA and NO (p < 0.05, HFD vs. NAR; 50 and 100 mg/kg) (partial prevention in the increase in MDA). Significant increase in SOD and GSH (p < 0.05, HFD vs. NAR; 50 and 100 mg/kg). |
| Antiobesogenic | Decreased weight gain (p < 0.05, HFD vs. NAR 100 mg/kg). Decreased epididymal and visceral adipose tissue (p < 0.05, HFD vs. NAR; 50 and 100 mg/kg) | |||
| Hormonal responses | Prevented increased plasma leptin significantly (p < 0.05, HFD vs. NAR; 50 and 100 mg/kg) Prevented decreased adiponectin in HFD (p < 0.05, HFD vs. NAR; 50 and 100 mg/kg) Increased hypothalamic STAT3 phosphorylation (p < 0.05, HFD vs. NAR; 50 and 100 mg/kg) |
| Hormone/Physiological Effect | Entero-Endocrine Cell Type | Localization by Highest Density | Main Receptor in Hunger–Satiety Pathway | Levels in Normal Weight | Levels in Obesity | Reported Effects of NAR |
|---|---|---|---|---|---|---|
| Ghrelin Orexigenic | P | Stomach | GHSR1A | Increase before meals, decrease after meals. | Low before meals and shorter duration of suppression after meals [83] | Ghrelin receptor is activated by NAR in vitro [84] |
| CCK Anorexigenic | I | Duodenum and proximal jejunum | CCK1 | Increase after meals, max. concentration 15 min. | Low after meals or failure to decrease after meals [85,86] | NAR stimulates CCK secretion in vitro [87] |
| GLP-1 Anorexigenic | L | Duodenum and colon | GLP-1 | Low before meals, high after meals. | Low before and after meals [88] | NR |
| PYY (3–36) Anorexigenic | L | Colon | Y2 | Increase after meals, max concentration 60–90 min | Variable. Some report low after meals [89] | NR |
| Insulin Anorexigenic | Pancreatic β-cells | Pancreas | IR | Low (compared with obese state) | High (in the insulin resistant state) | NAR enhances glucose-stimulated insulin secretion and glucose sensitivity in vitro [58] NAR improves insulin sensitivity, improves glucose and insulin tolerance in vivo through AMPK GLUT4 translocation [16] |
| Leptin Anorexigenic | Adipocyte | Adipose tissue | LepRb | Low (compared with obese state) | High [90] | NAR decreases serum leptin [19] and it′s expression in vivo [15] |
| Adiponectin Anorexigenic | Adipocyte | Adipose tissue | AdipoR1 and adipoR2 | High (compared with obese state) | Low (inversely proportional to adipose tissue mass) [91] | NAR increases serum adiponectin levels despite an HFD in vivo [19]. NAR enhances adiponectin mRNA expression in vivo [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Almada, G.; Domínguez-Avila, J.A.; Mejía-León, M.E.; Robles-Sánchez, M.; González-Aguilar, G.A.; Salazar-López, N.J. Could Naringenin Participate as a Regulator of Obesity and Satiety? Molecules 2023, 28, 1450. https://doi.org/10.3390/molecules28031450
López-Almada G, Domínguez-Avila JA, Mejía-León ME, Robles-Sánchez M, González-Aguilar GA, Salazar-López NJ. Could Naringenin Participate as a Regulator of Obesity and Satiety? Molecules. 2023; 28(3):1450. https://doi.org/10.3390/molecules28031450
Chicago/Turabian StyleLópez-Almada, Gabriela, J. Abraham Domínguez-Avila, María Esther Mejía-León, Maribel Robles-Sánchez, Gustavo A. González-Aguilar, and Norma Julieta Salazar-López. 2023. "Could Naringenin Participate as a Regulator of Obesity and Satiety?" Molecules 28, no. 3: 1450. https://doi.org/10.3390/molecules28031450
APA StyleLópez-Almada, G., Domínguez-Avila, J. A., Mejía-León, M. E., Robles-Sánchez, M., González-Aguilar, G. A., & Salazar-López, N. J. (2023). Could Naringenin Participate as a Regulator of Obesity and Satiety? Molecules, 28(3), 1450. https://doi.org/10.3390/molecules28031450