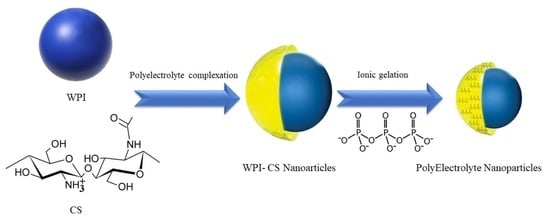
Whey Protein Isolate-Chitosan PolyElectrolyte Nanoparticles as a Drug Delivery System
Abstract
:1. Introduction
2. Results
2.1. Effect of pH and Concentration on Hydrodynamic Size
2.2. Morphology of PENs
2.3. Colloidal Stability Analysis
2.4. Infrared Spectrophotometry Analysis of PENs
2.5. Thermogravimetric Analysis
2.6. Drug Loading Assay of PENs
2.7. In Vitro Release Study of PENs
3. Materials and Methods
3.1. Materials
3.2. Preparation of PENs
3.3. Characterization of PENs
3.4. Drug Loading Assay of PENs
3.5. In Vitro Release Study of PENs
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [PubMed]
- Zegarra-Urquia, C.L.; Santiago, J.; Bumgardner, J.D.; Vega-Baudrit, J.; Hernández-Escobar, C.A.; Zaragoza-Contreras, E.A. Synthesis of nanoparticles of the chitosan-poly ((α, β)-DL-aspartic acid) polyelectrolite complex as hydrophilic drug carrier. Int. J. Polym. Mater. Polym. Biomater. 2022, 72, 497–506. [Google Scholar] [CrossRef]
- Teixeira, F.J.; Santos, H.O.; Howell, S.L.; Pimentel, G.D. Whey protein in cancer therapy: A narrative review. Pharmacol. Res. 2019, 144, 245–256. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Ghumman, S.A.; Mahmood, A.; Noreen, S.; Aslam, A.; Ijaz, B.; Amanat, A.; Kausar, R.; Rana, M.; Hameed, H. Chitosan-Linseed mucilage polyelectrolyte complex nanoparticles of Methotrexate: In vitro cytotoxic efficacy and toxicological studies. Arab. J. Chem. 2023, 16, 104463. [Google Scholar] [CrossRef]
- Motiei, M.; Aboutalebi, F.; Forouzanfar, M.; Dormiani, K.; Nasr-Esfahani, M.H.; Mirahmadi-Zare, S.Z. Smart co-delivery of miR-34a and cytotoxic peptides (LTX-315 and melittin) by chitosan based polyelectrolyte nanocarriers for specific cancer cell death induction. Mater. Sci. Eng. C 2021, 128, 112258. [Google Scholar] [CrossRef]
- Motiei, M.; Sedlařík, V.; Lucia, L.A.; Fei, H.; Münster, L. Stabilization of chitosan-based polyelectrolyte nanoparticle cargo delivery biomaterials by a multiple ionic cross-linking strategy. Carbohydr. Polym. 2020, 231, 115709. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Fattah Hassan, M.A.E. Preparation and Characterization of Sustained Released Zinc Citrate Encapsulated in Whey Protein Nanoparticles. Pak. J. Biol. Sci. PJBS 2018, 21, 448–453. [Google Scholar]
- McClements, D.J. Designing biopolymer microgels to encapsulate, protect and deliver bioactive components: Physicochemical aspects. Adv. Colloid Interface Sci. 2017, 240, 31–59. [Google Scholar] [CrossRef]
- Xu, W.; Tang, Y.; Yang, Y.; Wang, G.; Zhou, S. Establishment of a stable complex formed from whey protein isolate and chitosan and its stability under environmental stresses. Int. J. Biol. Macromol. 2020, 165, 2823–2833. [Google Scholar] [CrossRef]
- Cortés-Morales, E.A.; Mendez-Montealvo, G.; Velazquez, G. Interactions of the molecular assembly of polysaccharide-protein systems as encapsulation materials. A review. Adv. Colloid Interface Sci. 2021, 295, 102398. [Google Scholar] [CrossRef]
- Usman, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S.; Zia, F. Chitin and chitosan based polyurethanes: A review of recent advances and prospective biomedical applications. Int. J. Biol. Macromol. 2016, 86, 630–645. [Google Scholar] [CrossRef]
- Motiei, M.; Kashanian, S. Novel amphiphilic chitosan nanocarriers for sustained oral delivery of hydrophobic drugs. Eur. J. Pharm. Sci. 2017, 99, 285–291. [Google Scholar] [CrossRef]
- Speiciene, V.; Guilmineau, F.; Kulozik, U.; Leskauskaite, D. The effect of chitosan on the properties of emulsions stabilized by whey proteins. Food Chem. 2007, 102, 1048–1054. [Google Scholar] [CrossRef]
- Montilla, A.; Casal, E.; Moreno, F.J.; Belloque, J.; Olano, A.; Corzo, N. Isolation of bovine β-lactoglobulin from complexes with chitosan. Int. Dairy J. 2007, 17, 459–464. [Google Scholar] [CrossRef]
- Shu, X.; Zhu, K. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int. J. Pharm. 2000, 201, 51–58. [Google Scholar] [CrossRef]
- Diop, M.; Auberval, N.; Viciglio, A.; Langlois, A.; Bietiger, W.; Mura, C.; Peronet, C.; Bekel, A.; David, D.J.; Zhao, M. Design, characterisation, and bioefficiency of insulin–chitosan nanoparticles after stabilisation by freeze-drying or cross-linking. Int. J. Pharm. 2015, 491, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Motiei, M.; Mirahmadi-Zare, S.Z.; Nasr-Esfahani, M.H. Chemical stabilization of γ-polyglutamate by chitosan and the effect of co-solvents on the stability. Biophys. Chem. 2021, 275, 106605. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment. Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Gohargani, M.; Lashkari, H.; Shirazinejad, A. Study on biodegradable chitosan-whey protein-based film containing bionanocomposite TiO2 and Zataria multiflora essential oil. J. Food Qual. 2020, 2020, 8844167. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, X.; Ao, H.; Yin, Y.; Li, X.; Ren, D. Preparation and characterization of whey protein isolate/chitosan/microcrystalline cellulose composite films. Packag. Technol. Sci. 2021, 34, 589–599. [Google Scholar] [CrossRef]
- De Queiroz, J.L.C.; Costa, R.O.D.A.; Matias, L.L.R.; De Medeiros, A.F.; Gomes, A.F.T.; Pais, T.D.S.; Passos, T.S.; Maciel, B.L.L.; Dos Santos, E.A.; Morais, A.H.D.A. Chitosan-whey protein nanoparticles improve encapsulation efficiency and stability of a trypsin inhibitor isolated from Tamarindus indica L. Food Hydrocoll. 2018, 84, 247–256. [Google Scholar] [CrossRef]
- Xu, W.; Lv, K.; Mu, W.; Zhou, S.; Yang, Y. Encapsulation of α-tocopherol in whey protein isolate/chitosan particles using oil-in-water emulsion with optimal stability and bioaccessibility. LWT 2021, 148, 111724. [Google Scholar] [CrossRef]
- Aguiar, A.J.; de Queiroz, J.L.; Santos, P.P.; Camillo, C.S.; Serquiz, A.C.; Costa, I.S.; Oliveira, G.S.; Gomes, A.F.; Matias, L.L.; Costa, R.O.A.; et al. Beneficial Effects of Tamarind Trypsin Inhibitor in Chitosan–Whey Protein Nanoparticles on Hepatic Injury Induced High Glycemic Index Diet: A Preclinical Study. Int. J. Mol. Sci. 2021, 22, 9968. [Google Scholar] [CrossRef] [PubMed]
- Matias, L.L.; Costa, R.O.; Passos, T.S.; Queiroz, J.L.; Serquiz, A.C.; Maciel, B.L.; Santos, P.P.; Camillo, C.S.; Gonçalves, C.; Amado, I.R.; et al. Tamarind trypsin inhibitor in chitosan–whey protein nanoparticles reduces fasting blood glucose levels without compromising insulinemia: A preclinical study. Nutrients 2019, 11, 2770. [Google Scholar] [CrossRef]
- Lin, C.; Kuo, T.-C.; Lin, J.-C.; Ho, Y.-C.; Mi, F.-L. Delivery of polysaccharides from Ophiopogon japonicus (OJPs) using OJPs/chitosan/whey protein co-assembled nanoparticles to treat defective intestinal epithelial tight junction barrier. Int. J. Biol. Macromol. 2020, 160, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-Y.; Lin, J.-W.; Wang, R.; Chen, B.-R.; Li, J.; Wen, Q.-H.; Zeng, X.-A. Succinylated whey protein isolate-chitosan core–shell composite particles as a novel carrier: Self-assembly mechanism and stability studies. Food Res. Int. 2022, 160, 111695. [Google Scholar] [CrossRef]
- Alves, A.C.; Magarkar, A.; Horta, M.; Lima, J.L.; Bunker, A.; Nunes, C.; Reis, S. Influence of doxorubicin on model cell membrane properties: Insights from in vitro and in silico studies. Sci. Rep. 2017, 7, 6343. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Ferreira, S.O.; de Alvarenga, E.S.; Soares, N.d.F.F.; dos Reis Coimbra, J.S.; de Oliveira, E.B. Polyelectrolyte complexes (PECs) obtained from chitosan and carboxymethylcellulose: A physicochemical and microstructural study. Carbohydr. Polym. Technol. Appl. 2022, 3, 100197. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Ramesh, K.; Kumar, D.N.; Dehari, D.; Singh, S.; Kumar, D.; Agrawal, A.K. Polymeric micelles: A novel drug delivery system for the treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2022, 77, 103886. [Google Scholar] [CrossRef]
- Motiei, M.; Kashanian, S.; Lucia, L.A.; Khazaei, M. Intrinsic parameters for the synthesis and tuned properties of amphiphilic chitosan drug delivery nanocarriers. J. Control. Release 2017, 260, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Sultan, M.H.; Moni, S.S.; Madkhali, O.A.; Bakkari, M.A.; Alshahrani, S.; Alqahtani, S.S.; Alhakamy, N.A.; Mohan, S.; Ghazwani, M.; Bukhary, H.A. Characterization of cisplatin-loaded chitosan nanoparticles and rituximab-linked surfaces as target-specific injectable nano-formulations for combating cancer. Sci. Rep. 2022, 12, 468. [Google Scholar] [CrossRef]
- Hecq, J.; Siepmann, F.; Siepmann, J.; Amighi, K.; Goole, J. Development and evaluation of chitosan and chitosan derivative nanoparticles containing insulin for oral administration. Drug Dev. Ind. Pharm. 2015, 41, 2037–2044. [Google Scholar] [CrossRef]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Paramakrishnan, N.; Suresh, B. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly (n-butylcyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2008, 70, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Sinha, P.; Laha, B.; Maiti, S.; Bhattacharyya, U.K.; Nayak, A.K. Polysorbate 80 coated crosslinked chitosan nanoparticles of ropinirole hydrochloride for brain targeting. J. Drug Deliv. Sci. Technol. 2018, 48, 21–29. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2013; Volume 2. [Google Scholar]
- Huang, G.-Q.; Sun, Y.-T.; Xiao, J.-X.; Yang, J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012, 135, 534–539. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Delair, T. Stabilization of chitosan/hyaluronan colloidal polyelectrolyte complexes in physiological conditions. Carbohydr. Polym. 2015, 119, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Verheul, R.J.; Slütter, B.; Bal, S.M.; Bouwstra, J.A.; Jiskoot, W.; Hennink, W.E. Covalently stabilized trimethyl chitosan-hyaluronic acid nanoparticles for nasal and intradermal vaccination. J. Control. Release 2011, 156, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qineng, P.; Zhang, H. Self-assembly and characterization of paclitaxel-loaded N-octyl-O-sulfate chitosan micellar system. Colloids Surf. B Biointerfaces 2004, 39, 69–75. [Google Scholar] [CrossRef]
- Dragan, E.S.; Ghiorghita, C.A.; Dinu, M.V.; Duceac, I.A.; Coseri, S. Fabrication of self-antibacterial chitosan/oxidized starch polyelectrolyte complex sponges for controlled delivery of curcumin. Food Hydrocoll. 2023, 135, 108147. [Google Scholar] [CrossRef]
- Mattu, C.; Li, R.; Ciardelli, G. Chitosan nanoparticles as therapeutic protein nanocarriers: The effect of pH on particle formation and encapsulation efficiency. Polym. Composites. 2013, 34, 1538–1545. [Google Scholar] [CrossRef]





| Day | Z-Average Size | PDI |
|---|---|---|
| 1 | 248.57 ± 5.00 a | 0.41 ± 0.02 e |
| 8 | 253.10 ± 6.21 b | 0.44 ± 0.06 f |
| 15 | 261.20 ± 8.35 c | 0.46 ± 0.05 g |
| 22 | 261.87 ± 9.16 d | 0.49 ± 0.04 h |
| 29 | 345.03 ± 44.64 abcd | 0.80 ± 0.21 efgh |
| Compounds | Total Loss of Mass (%) |
|---|---|
| CS | 65.87 |
| TPP | 1.456 |
| WPI | 77.48 |
| PENs | 79.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadollahi, Z.; Motiei, M.; Kazantseva, N.; Císař, J.; Sáha, P. Whey Protein Isolate-Chitosan PolyElectrolyte Nanoparticles as a Drug Delivery System. Molecules 2023, 28, 1724. https://doi.org/10.3390/molecules28041724
Yadollahi Z, Motiei M, Kazantseva N, Císař J, Sáha P. Whey Protein Isolate-Chitosan PolyElectrolyte Nanoparticles as a Drug Delivery System. Molecules. 2023; 28(4):1724. https://doi.org/10.3390/molecules28041724
Chicago/Turabian StyleYadollahi, Zahra, Marjan Motiei, Natalia Kazantseva, Jaroslav Císař, and Petr Sáha. 2023. "Whey Protein Isolate-Chitosan PolyElectrolyte Nanoparticles as a Drug Delivery System" Molecules 28, no. 4: 1724. https://doi.org/10.3390/molecules28041724
APA StyleYadollahi, Z., Motiei, M., Kazantseva, N., Císař, J., & Sáha, P. (2023). Whey Protein Isolate-Chitosan PolyElectrolyte Nanoparticles as a Drug Delivery System. Molecules, 28(4), 1724. https://doi.org/10.3390/molecules28041724