An Interesting Conversion Route of Mononuclear Zinc Complex to Zinc Mixed Carboxylate Coordination Polymer
Abstract
:1. Introduction
2. Results and Discussion
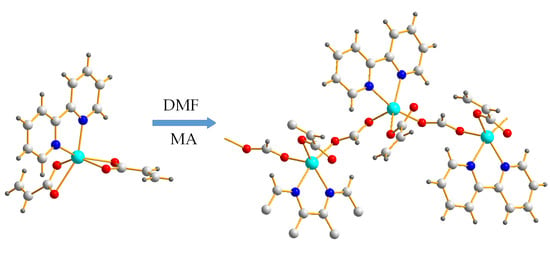
2.1. Synthesis of the Complex
2.2. X-ray Crystal Structure of Complex
2.3. IR Spectra
2.4. Thermal Behavior
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Syntheses of Precursor and Coordination Polymer
3.3. X-ray Crystal Structure Analysis of 1a
4. Conclusions and Further Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Driessen, W.; Chang, L.; Finazzo, C.; Gorter, S.; Rehost, D.; Reedijk, J.; Lutz, M.; Spek, A. Two pyrazolato-bridged, linear trinuclear Cu(II) complexes. Crystal structures and magnetic properties. Inorg. Chim. Acta 2003, 350, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.C.; Roh, J.; Lough, A. Isolation and crystal structure of [Cu(L1)(O2CH)](ClO4)∙H2O]: Hydrolysis of DMF (N,N-dimethylformamide) by a copper(II) tetraazamacrocycle. J. Chem. Crystallogr. 2007, 37, 615–618. [Google Scholar] [CrossRef]
- Schepartz, A.; Breslow, R. Hydrolysis of an amide in a carboxypeptidase model using Co(III) and bifunctional catalysts. J. Am. Chem. Soc. 1987, 109, 1814–1826. [Google Scholar] [CrossRef]
- Kreżel, A.; Mylonas, M.; Kopera, E.; Bal, W. Sequence-specific Ni(II)-dependent peptide bond hydrolysis in a peptide containing threonine and histidine residues. Acta Biochim. Pol. 2006, 53, 721–727. [Google Scholar] [CrossRef]
- Fife, T.; Przystas, T. Divalent metal ion catalysis in amide hydrolysis. The hydrolysis of N-acylimidazoles. J. Am. Chem. Soc. 1986, 108, 4631–4636. [Google Scholar] [CrossRef]
- Huang, G.; Yang, P.; Wang, N.; Wu, J.-Z.; Yu, Y. First lanthanide coordination polymers with N,N-dimethylformamide hydrolysis induced formate ligands. Inorg. Chim. Acta 2012, 384, 333–339. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, N.; Xiao, W.; Chen, C. Crystal structure of neodymium (III) formate, Nd(HCO2)3. Z. Kristallogr. NCS 2009, 224, 275–276. [Google Scholar] [CrossRef]
- Namli, H.; Azaz, A.D.; Karabulut, S.; Çelen, S.; Kurtaran, R. Synthesis, characterization, crystal structure and biological activity of the cobalt (IV) complex of 2,6-diacetylpyridine dioxime: [Co(dapdo)2]. Trans. Met. Chem. 2007, 32, 266–270. [Google Scholar] [CrossRef]
- Yildirim, L.T.; Kurtaran, R.; Namli, H.; Azaz, A.D.; Atakol, O. Synthesis, crystal structure and biological activity of two new heterotrinuclearthiocyanato bridged Cu(II)-Hg(II)-Cu(II) complexes. Polyhedron 2007, 26, 4187–4194. [Google Scholar] [CrossRef]
- Suzuki, H.; Ishiguro, S.-I. N,N-Dimethylformamide complex of aluminium (III) perclorate. Acta Crystallogr. 1998, C54, 586–588. [Google Scholar] [CrossRef]
- Wu, H.; Yuan, J.; Qi, B.; Kong, J.; Kou, F.; Jia, F.; Fan, X.; Wang, Y. A seven-coordinate manganese (II) complex formed with tripodal tetradentate ligand tris (N-methylbenzimidazol-2-ylmethyl)amine. Z. Naturforsch. 2010, 65b, 1097–1100. [Google Scholar] [CrossRef]
- Bauer, C.; Timofeeva, T.; Settersten, T.; Patterson, B.; Liu, V.; Simmons, B.; Allendorf, M. Influence of connectivity and porosity on ligand-based luminiscence in zinc metal-organic frameworks. J. Am. Chem. Soc. 2007, 129, 7136–7144. [Google Scholar] [CrossRef]
- Sobieray, M.; Gode, J.; Seidel, C.; Poss, M.; Feldmann, C.; Ruschewitz, U. Bright luminiscence in lanthanide coordination polymers with tetrafluoroterephtalate as bridging ligand. Dalton Trans. 2015, 44, 6249–6259. [Google Scholar] [CrossRef]
- Kurtaran, R.; Yildirim, L.T.; Azaz, A.D.; Namli, H.; Atakol, O. Synthesis, characterization, crystal structure and biological activity of a novel heterotetranuclear complex: [NiLPb(SCN)2(DMF)(H2O)]2, bis-{[μ-N,N′-bis(salicylidene)-1,3-propanediaminato-aqua-nickel(II)](thiocyanato)(μ-thiocyanato)(μ-N,N′-dimethylformamide)lead(II)}. J. Inorg. Chem. 2005, 99, 1937–1944. [Google Scholar] [CrossRef]
- Neofotistou, E.; Malliakas, C.; Trikalitis, P. Novel coordination polymers based on the tetrathioterephtalate dianion as the bridging ligand. Inorg. Chem. 2007, 46, 8487–8489. [Google Scholar] [CrossRef]
- Wei, S.Y.; Bai, F.Y.; Song, G.; Hou, Y.N.; Xu, X.T.; Zhang, X.X.; Zhang, H.Z.; Xing, Y.H. Design, synthesis, structural and spectra characterize of metal (II) formate complexes [M(O2CH)2]∙n(Solvent) (M = Mn, Cu). Commun. Inorg. Synth. 2014, 2, 5–10. [Google Scholar] [CrossRef]
- Tambornino, F.; Hoch, C. Crystal structure of the dodecanuclear coordination compounds [RE12(DMF)24(HCOO)8(OH)16]I3·4DMF (RE = Eu, Nd). Aust. J. Chem. 2022, 75, 587–594. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Zhai, H.; Li, D. Hydrolysis of N,N-dimethylformamide catalyzed by the Keggin H3[PMo12O40]: Isolation and crystal structure analysis of [(CH3)2NH2]3[PMo12O40]. J. Mol. Struct. 2003, 654, 215–221. [Google Scholar] [CrossRef]
- Hawmaxwell, S.M.; Brammer, L. Solvent hydrolysis leads to an unusual Cu(II) metal-organic framework. Cryst. Eng. Comm. 2006, 8, 473–476. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.-Y.; Chen, C.; Yue, Q.; Yuan, H.-M.; Chen, J.-S.; Wang, S.-N. Photoluminiscent metal-organic polymer constructed from trimetallic clusters and mixed carboxylates. Inorg. Chem. 2003, 42, 944–946. [Google Scholar] [CrossRef]
- Burrows, A.; Cassar, K.; Friend, R.; Mahon, M.; Rigby, S.; Warren, J. Solvent hydrolysis and templating effects in the synthesis of metal-organic frameworks. CrystEngComm 2005, 7, 548–550. [Google Scholar] [CrossRef]
- Ganesan, S.V.; Lightfoot, P.; Natarajan, S. A new zinc pyromellitate, [(C4N2H12)0.5(NH2(CH3)2)][Zn(C10H2O8)]∙1.78H2O, a layered structure. Solid State Sci. 2004, 6, 757–762. [Google Scholar] [CrossRef]
- Zhu, Z.; Meng, X.-M.; Zhang, D.-M.; Zhang, X.; Wang, M.; Jin, F.; Fan, Y.-H. Syntheses, structures and selective dye adsorption of five formic-based coordination polymers prepared by in-situ hydrolysis of N,N′-dimethylformamide. J. Solid State Chem. 2017, 248, 109–118. [Google Scholar] [CrossRef]
- Vasile Scăețeanu, G.; Chifiriuc, M.; Bleotu, C.; Kamerzan, C.A.; Măruţescu, L.; Daniliuc, C.; Maxim, C.; Calu, L.; Olar, R.; Badea, M. Synthesis, structural characterization, antimicrobial activity, and in vitro biocompatibility of new unsaturated carboxylate complexes with 2,2′-bipyridine. Molecules 2018, 23, 157. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, R.C.; Parashar, G.K. Synthesis, molecular weights and infrared spectra of mixed carboxylate complexes of titanium (IV). Synth. React. Inorg. Met. Org. Chem. 1978, 8, 195–205. [Google Scholar] [CrossRef]
- Palaniandavar, M. Carboxylate mixed ligand complexes of copper (II) with some chelating carbonyl and imine ligands and their reactivity. Trans. Met. Chem. 1983, 8, 14–16. [Google Scholar] [CrossRef]
- Emori, S.; Yoshidzu, K. Preparation and magnetic properties of copper (II) mixed carboxylates with haloacetate and ammonioacetate. Bull. Chem. Soc. Jpn. 1991, 64, 301–302. [Google Scholar] [CrossRef]
- Khokhar, A.; Deng, Y.; Kido, Y.; Siddik, Z. Preparation, characterization and antitumor activity of new ethylenediamine platinum (IV) complexes containing mixed carboxylate ligands. J. Inorg. Biochem. 1993, 50, 79–87. [Google Scholar] [CrossRef]
- Soler, M.; Artus, P.; Folting, K.; Huffman, J.; Hendrickson, D.; Christou, G. Single-molecule magnets: Preparation and properties of mixed-carboxylate complexes [Mn12O12(O2CR)8(O2CR′)8(H2O)4]. Inorg. Chem. 2001, 40, 4902–4912. [Google Scholar] [CrossRef] [Green Version]
- Stamatatos, T.; Katsoulakoua, E.; Nastopoulos, V.; Raptopoulou, C.; Manessi-Zoupa, E. Cadmium carboxylate chemistry: Preparation, crystal structure and thermal and spectroscopic characterization of the one-dimensional polymer [Cd(O2CMe)(O2CPh)(H2O)2]n. Z. Naturforsch. 2003, 58b, 1045–1054. [Google Scholar] [CrossRef]
- Soldevila-Sanmartin, J.; Ayllon, J.; Calvet, T.; Font-Bardia, M.; Domingo, C.; Pons, J. Synthesis, crystal structure and magnetic properties of a Cu(II) paddle-wheel complex with mixed bridges. Inorg. Chem. Commun. 2016, 71, 90–93. [Google Scholar] [CrossRef] [Green Version]
- Adimula, V.; Tella, A.; Udeaja, I.; Durodoye, A.; Lawal, A. Coordination polymers based on mixed carboxylate ligands: Synthesis and thermal studies. Commun. Fac. Sci. Univ. Ank. Ser. 2018, 60, 1–16. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Youngme, S.; Phatchimkun, J.; Suksangpanya, U.; Pakawatchai, C.; Chaichit, N.; Kongsaeree, P.; Krzystek, J.; Murphy, B. Crystal structures, spectro-structural correlation and structural pathways of eight [Cu(dpyam)2(NCO)][Y] complexes (dpyam = di-2-pyridylamine), Y = Br−, CF3SO3−, BF4−·dpyam, Cl−·4H2O, NO3−, PF6−, ClO4− and BPh4−. Polyhedron 2007, 26, 871–882. [Google Scholar] [CrossRef]
- Liu, J.; Kumar, A.; Singh, A.; Jin, J.; Deng, F.; Wu, X.; Luo, Z. Luminescent sensing and photocatalytic degradation properties of an uncommon (4,5,5)-connected 3D MOF based on 3,5-di(3′,5′-dicarboxylphenyl)benzoic acid: An experimental and computational study. CrystEngComm 2017, 19, 4368–4377. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Oldham, C. Carboxylates, squarates and related species. In Comprehensive Coordination Chemistry, 1st ed.; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; pp. 435–460. [Google Scholar]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J. A new 3D 8-connected Cd(II) MOF as a potent photocatalyst for oxytetracycline antibiotic degradation. CrystEngComm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Micropor. Mesopor. Mat. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Deacon, G.B.; Philips, J.R. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Bruker. APEX3 Version 2016.1-0, SAINT Version 8-37A and SADABS Version 2014/7; Bruker AXS Inc.: Madison, WI, USA.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- XP—Interactive Molecular Graphics, Version 5.1; Bruker AXS Inc.: Madison, WI, USA, 1998.





| D-H⋯A | d(D-H) | d(H⋯A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| C9A-H9A⋯O4B #1 | 0.94 | 2.526 | 3.456 | 170.0 |
| C10A-H10A⋯O3A | 0.94 | 2.506 | 3.075 | 119.1 |
| C14A-H14A⋯O1A #2 | 0.94 | 2.423 | 3.068 | 125.7 |
| C14A-H14A⋯O2A | 0.94 | 2.492 | 3.212 | 133.5 |
| C1B-H1B⋯O4B | 0.94 | 2.528 | 3.077 | 117.5 |
| C2B-H2B⋯O3A #2 | 0.94 | 2.532 | 3.426 | 159.1 |
| C14B-H14B⋯O1B | 0.94 | 2.435 | 3.078 | 125.5 |
| C14B-H14B⋯O2B #3 | 0.94 | 2.446 | 3.185 | 135.5 |
| Cg1⋯Cg1 #4 | 3.537 |
| (1) | (1a) | Assignments |
|---|---|---|
| 1643 vs | 1639 vs | ν(C=C) (acrylato ion) |
| 1551 vs | 1619 vs 1580 vs | νas(COO) |
| 1492 m | 1475 m | ν(C=C) + ν(C=N) (bpy) |
| 1443 vs | 1435 s | δ(CH) |
| 1388 s | 1392 vs 1366 s | νs(COO) |
| 689 m | 678 m | δ(COO) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile Scăețeanu, G.; Daniliuc, C.G.; Olar, R.; Badea, M. An Interesting Conversion Route of Mononuclear Zinc Complex to Zinc Mixed Carboxylate Coordination Polymer. Molecules 2023, 28, 2011. https://doi.org/10.3390/molecules28052011
Vasile Scăețeanu G, Daniliuc CG, Olar R, Badea M. An Interesting Conversion Route of Mononuclear Zinc Complex to Zinc Mixed Carboxylate Coordination Polymer. Molecules. 2023; 28(5):2011. https://doi.org/10.3390/molecules28052011
Chicago/Turabian StyleVasile Scăețeanu, Gina, Constantin G. Daniliuc, Rodica Olar, and Mihaela Badea. 2023. "An Interesting Conversion Route of Mononuclear Zinc Complex to Zinc Mixed Carboxylate Coordination Polymer" Molecules 28, no. 5: 2011. https://doi.org/10.3390/molecules28052011
APA StyleVasile Scăețeanu, G., Daniliuc, C. G., Olar, R., & Badea, M. (2023). An Interesting Conversion Route of Mononuclear Zinc Complex to Zinc Mixed Carboxylate Coordination Polymer. Molecules, 28(5), 2011. https://doi.org/10.3390/molecules28052011