Sol-Gel Graphene Oxide-Coated Fabric Disks as Sorbents for the Automatic Sequential-Injection Column Preconcentration for Toxic Metal Determination in Distilled Spirit Drinks
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Sol-Gel Graphene Oxide-Coated Polyester Fabric Membranes
2.2. Optimization of the On-Line Column Preconcentration Procedure
2.2.1. Effect of Chelating Agent and Sample Acidity
2.2.2. Effect of Loading Flow Rate and Preconcentration Time
2.2.3. Effect of Eluent Type and Elution Flow Rate
2.2.4. Effect of Sample Volume Injected in Graphite Furnace
2.2.5. Effect of Sample Ethanol Content
2.3. Figures of Merit
2.4. Interferences Studies
2.5. Real Sample Analysis
2.6. Comparison with Other Studies
2.7. Evaluation of the Green Character of the Proposed Method
3. Materials and Methods
3.1. Reagents, Materials and Samples
3.2. Instrumentation
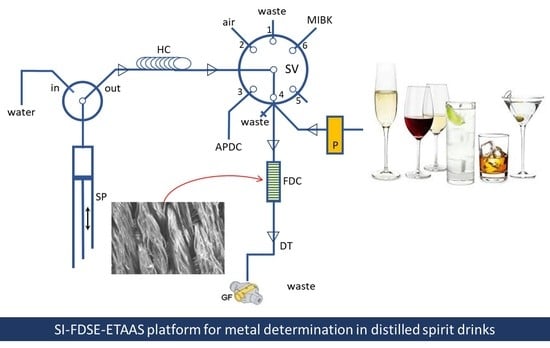
3.3. Fabrication of the FDSE Microcolumn
3.4. On-Line FDSE Analytical Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ibanez, J.G.; Carreon-Alvarez, A.; Barcena-Soto, M.; Casillas, N. Metals in alcoholic beverages: A review of sources, effects, concentrations, removal, speciation, and analysis. J. Food Compos. Anal. 2008, 21, 672–683. [Google Scholar] [CrossRef]
- Tobiasz, A.; Walas, S. Solid-phase-extraction procedures for atomic spectrometry determination of copper. TrAC-Trends Anal. Chem. 2014, 62, 106–122. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Khalili, N.; Rezaei, N. Effects of lead and cadmium on the immune system and cancer progression. J. Environ. Health Sci. Eng. 2020, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Miró, M.; Estela, J.M.; Cerdà, V. Application of flowing stream techniques to water analysis: Part III. Metal ions: Alkaline and alkaline-earth metals, elemental and harmful transition metals, and multielemental analysis. Talanta 2004, 63, 201–223. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bezerra, M.A.; Santos, A.S.; dos Santos, W.N.L.; Novaes, C.G.; de Oliveira, O.M.C.; Oliveira, M.L.; Garcia, R.L. Atomic absorption spectrometry—A multi element technique. TrAC-Trends Anal. Chem. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Giakisikli, G.; Anthemidis, A.N. An automatic stirring-assisted liquid–liquid microextraction system based on lab-in-syringe platform for on-line atomic spectrometric determination of trace metals. Talanta 2017, 166, 364–368. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC-Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Manousi, N.; Kabir, A.; Furton, K.G.; Stathogiannopoulou, M.; Drosaki, E.; Anthemidis, A. An automatic on-line sol-gel pyridylethylthiopropyl functionalized silica-based sorbent extraction system coupled to flame atomic absorption spectrometry for lead and copper determination in beer samples. Food Chem. 2022, 394, 133548. [Google Scholar] [CrossRef]
- Ribeiro, G.C.; Coelho, L.M.; Coelho, N.M.M. Determination of nickel in alcoholic beverages by faas after online preconcentration using Mandarin Peel (Citrus reticulata) as biosorbent. J. Braz. Chem. Soc. 2013, 24, 1072–1078. [Google Scholar] [CrossRef]
- Kabir, A.; Samanidou, V. Fabric Phase Sorptive Extraction: A Paradigm Shift Approach in Analytical and Bioanalytical Sample Preparation. Molecules 2021, 26, 865. [Google Scholar] [CrossRef]
- Manousi, N.; Kabir, A.; Zachariadis, G.A. Green bioanalytical sample preparation: Fabric phase sorptive extraction. Bioanalysis 2021, 13, 693–710. [Google Scholar] [CrossRef]
- Kazantzi, V.; Anthemidis, A. Fabric sol–gel phase sorptive extraction technique: A review. Separations 2017, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Anthemidis, A.; Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G. An automated flow injection system for metal determination by flame atomic absorption spectrometry involving on-line fabric disk sorptive extraction technique. Talanta 2016, 156–157, 64–70. [Google Scholar] [CrossRef]
- Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G.; Anthemidis, A. On-line fabric disk sorptive extraction via a flow preconcentration platform coupled with atomic absorption spectrometry for the determination of essential and toxic elements in biological samples. Separations 2018, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Celeiro, M.; Acerbi, R.; Kabir, A.; Furton, K.G.; Llompart, M. Development of an analytical methodology based on fabric phase sorptive extraction followed by gas chromatography-tandem mass spectrometry to determine UV filters in environmental and recreational waters. Anal. Chim. Acta X 2020, 4, 100038. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Ampatzi, N.; Kabir, A.; Furton, K.G.; Samanidou, V.F. Development of a capsule phase microextraction methodology for the selective determination of coumarin in foodstuff analyzed by HPLC-DAD. Adv. Sample Prep. 2022, 3, 100026. [Google Scholar] [CrossRef]
- Mazaraki, K.; Kabir, A.; Furton, K.G.; Fytianos, K.; Samanidou, V.F.; Zacharis, C.K. Fast fabric phase sorptive extraction of selected β-blockers from human serum and urine followed by UHPLC-ESI-MS/MS analysis. J. Pharm. Biomed. Anal. 2021, 199, 114053. [Google Scholar] [CrossRef]
- Dvořák, M.; Miró, M.; Kubáň, P. Automated Sequential Injection-Capillary Electrophoresis for Dried Blood Spot Analysis: A Proof-of-Concept Study. Anal. Chem. 2022, 94, 5301–5309. [Google Scholar] [CrossRef]
- Horstkotte, B.; Miró, M.; Solich, P. Where are modern flow techniques heading to? Anal. Bioanal. Chem. 2018, 410, 6361–6370. [Google Scholar] [CrossRef]
- Zacharis, C.K.; Theodoridis, G.A.; Voulgaropoulos, A.N. Coupling of sequential injection with liquid chromatography for the automated derivatization and on-line determination of amino acids. Talanta 2006, 69, 841–847. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.A.; Zachariadis, G.A. Sample preparation using graphene-oxide-derived nanomaterials for the extraction of metals. Molecules 2020, 25, 2411. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liang, Q.; Han, Q.; Zhang, X.; Ding, M. One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta 2015, 132, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.L.; Chen, F.S.; Zhang, J.; Ren, S.B.; Liang, H.D.; Li, S.S. On-line flame AAS determination of traces Cd(II) and Pb(II) in water samples using thiol-functionalized SBA-15 as solid phase extractant. J. Ind. Eng. Chem. 2015, 27, 362–367. [Google Scholar] [CrossRef]
- Anthemidis, A.; Tzili, A. An automatic on-line sorptive extraction platform for palladium determination in automobile exhaust catalysts based on a PTFE-turnings packed column, flow injection analysis and flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2021, 1–13. [Google Scholar] [CrossRef]
- Rascón, A.J.; Azzouz, A.; Ballesteros, E. Use of semi-automated continuous solid-phase extraction and gas chromatography–mass spectrometry for the determination of polycyclic aromatic hydrocarbons in alcoholic and non-alcoholic drinks from Andalucía (Spain). J. Sci. Food Agric. 2019, 99, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Wenzl, T. Determination of bisphenols in beverages by mixed-mode solid-phase extraction and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1422, 230–238. [Google Scholar] [CrossRef]
- Mena, C.; Cabrera, C.; Lorenzo, M.L.; López, M.C. Cadmium levels in wine, beer and other alcoholic beverages: Possible sources of contamination. Sci. Total Environ. 1996, 181, 201–208. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A.; Overah, L.C.; Bassey, F.I.; Martincigh, B.S. Trace metal concentrations in distilled alcoholic beverages and liquors in Nigeria. J. Instig. Brew. 2014, 120, 521–528. [Google Scholar] [CrossRef]
- Barbeira, P.J.S.; Stradiotto, N.R. Simultaneous determination of trace amounts of zinc, lead and copper in rum by anodic stripping voltammetry. Talanta 1997, 44, 185–188. [Google Scholar] [CrossRef]
- Tatarková, M.; Baška, T.; Ulbrichtová, R.; Kuka, S.; Sovičová, M.; Štefanová, E.; Malobická, E.; Hudečková, H. Determination of Cadmium and Chromium in Fruit Spirits Intended for Own Consumption Using Graphite Furnace Atomic Absorption Spectrometry. Acta Med. 2021, 64, 213–217. [Google Scholar] [CrossRef]
- Mena, C.M.; Cabrera, C.; Lorenzo, M.L.; Lopez, M.C. Determination of Lead Contamination in Spanish Wines and Other Alcoholic Beverages by Flow Injection Atomic Absorption Spectrometry. J. Agric. Food Chem. 1997, 45, 1812–1815. [Google Scholar] [CrossRef]
- Galani-Nikolakaki, S.; Kallithrakas-Kontos, N.; Katsanos, A.A. Trace element analysis of Cretan wines and wine products. Sci. Total Environ. 2002, 285, 155–163. [Google Scholar] [CrossRef]
- Sampaio, O.M.; Reche, R.V.; Franco, D.W. Chemical profile of rums as a function of their origin. The use of chemometric techniques for their identification. J. Agric. Food Chem. 2008, 56, 1661–1668. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Andrade-Sobrinho, L.G.; Leite-Neto, A.F.; Reche, R.V.; Isique, W.D.; Ferreira, M.M.C.; Lima-Neto, B.S.; Franco, D.W. Comparison between cachaça and rum using pattern recognition methods. J. Agric. Food Chem. 2004, 52, 3429–3433. [Google Scholar] [CrossRef]
- Elçi, L.; Arslan, Z.; Tyson, J.F. Determination of lead in wine and rum samples by flow injection-hydride generation-atomic absorption spectrometry. J. Hazard. Mater. 2009, 162, 880–885. [Google Scholar] [CrossRef] [Green Version]
- Anthemidis, A.N.; Ioannou, K.I.G. Development of a sequential injection dispersive liquid-liquid microextraction system for electrothermal atomic absorption spectrometry by using a hydrophobic sorbent material: Determination of lead and cadmium in natural waters. Anal. Chim. Acta 2010, 668, 35–40. [Google Scholar] [CrossRef]
- Giakisikli, G.; Anthemidis, A.N. Automated magnetic sorbent extraction based on octadecylsilane functionalized maghemite magnetic particles in a sequential injection system coupled with electrothermal atomic absorption spectrometry for metal determination. Talanta 2013, 110, 229–235. [Google Scholar] [CrossRef]
- Miró, M.; Jończyk, S.; Wang, J.; Hansen, E.H. Exploiting the bead-injection approach in the integrated sequential injection lab-on-valve format using hydrophobic packing materials for on-line matrix removal and preconcentration of trace levels of cadmium in environmental and biological samples via fomation of non-charged chelates prior to ETAAS detection. J. Anal. At. Spectrom. 2003, 18, 89–98. [Google Scholar] [CrossRef]
- Chen, X.W.; Huang, L.L.; He, R.H. Silk fibroin as a sorbent for on-line extraction and preconcentration of copper with detection by electrothermal atomic absorption spectrometry. Talanta 2009, 78, 71–75. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Adam, I.S.I. Development of on-line single-drop micro-extraction sequential injection system for electrothermal atomic absorption spectrometric determination of trace metals. Anal. Chim. Acta 2009, 632, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Vereda Alonso, E.; Siles Cordero, M.T.; García De Torres, A.; Cano Pavón, J.M. Lead ultra-trace on-line preconcentration and determination using selective solid phase extraction and electrothermal atomic absorption spectrometry: Applications in seawaters and biological samples. Anal. Bioanal. Chem. 2006, 385, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hansen, E.H. Development of an automated sequential injection on-line solvent extraction-back extraction procedure as demonstrated for the determination of cadmium with detection by electrothermal atomic absorption spectrometry. Anal. Chim. Acta 2002, 456, 283–292. [Google Scholar] [CrossRef]
- Yu, Y.L.; Du, Z.; Wang, J.H. Determination of Copper in Seawater Using a Sequential Injection System Incorporating a Sample Pretreatment Module Coupled to Electrothermal Atomic Absorption Spectrometry. Chin. J. Anal. Chem. 2007, 35, 431–434. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC-Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Samanidou, V.; Filippou, O.; Marinou, E.; Kabir, A.; Furton, K.G. Sol–gel-graphene-based fabric-phase sorptive extraction for cow and human breast milk sample cleanup for screening bisphenol A and residual dental restorative material before analysis by HPLC with diode array detection. J. Sep. Sci. 2017, 40, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Parvinzadeh, M.; Ebrahimi, I. Influence of atmospheric-air plasma on the coating of a nonionic lubricating agent on polyester fiber. Radiat. Eff. Defects Solids 2011, 166, 408–416. [Google Scholar] [CrossRef]




| Parameter | Cd(II) | Cu(II) | Pb(II) |
|---|---|---|---|
| Slope, S (μg L−1) | 2.3000 ± 0.1004 | 0.6272 ± 0.0256 | 0.2568 ± 0.0078 |
| Intercept, i | 0.0030 ± 0.0093 | 0.0022 ± 0.0100 | 0.0033 ± 0.0055 |
| Linear range (μg L−1) | 0.0064–0.160 | 0.0236–0.70 | 0.0578–1.5 |
| Correlation coefficient, r2 | 0.9985 | 0.9987 | 0.9993 |
| LOD (ng L−1) | 1.9 | 7.1 | 17.3 |
| LOQ (ng L−1) | 6.4 | 23.6 | 57.8 |
| RSD (%) | 2.4 (0.02 μg L−1) | 2.2 (0.10 μg L−1) | 2.9 (0.25 μg L−1) |
| Preconcentration time (s) | 90 | 90 | 90 |
| Sample throughput (h−1) | 24 | 24 | 24 |
| Enhancement factor | 38 | 120 | 85 |
| Sample consumption (mL) | 13.5 | 13.5 | 13.5 |
| Certified Reference Material | Cd | Cu | Pb |
|---|---|---|---|
| CRM 1643e (Trace Element Water) | |||
| Certified value (μg L−1) | 6.568 ± 0.073 | 22.76 ± 0.31 | 19.63 ± 0.21 |
| Found (μg L−1) | 6.35 ± 0.29 | 22.26 ± 0.66 | 18.9 ± 1.12 |
| Relative Error | −3.3 | −2.2 | −3.7 |
| texp | 1.302 | 1.312 | 1.129 |
| IAEA-433 (Marine sediment) | |||
| Certified value (μg L−1) | 0.153 ± 0.033 | 30.8 ± 2.6 | 26.0 ± 2.7 |
| Found (μg L−1) | 0.149 ± 0.015 | 29.5 ± 1.2 | 26.4 ± 1.8 |
| Relative Error | −2.6 | −4.2 | 1.5 |
| texp | 0.462 | 1.876 | −0.385 |
| BCR 278-R (Mussel tissue) | |||
| Certified value (μg L−1) | 0.348 ± 0.007 | 9.45 ± 0.13 | 2.00 ± 0.04 |
| Found (μg L−1) | 0.33 ± 0.02 | 9.32 ± 0.35 | 1.90 ± 0.09 |
| Relative Error | −5.2 | −1.4 | −5.0 |
| texp | 1.559 | 0.643 | 1.925 |
| Sample | Cd | Cu 1 | Pb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Added (μg L−1) | Found (μg L−1) | Recovery (%) | texp | Added (μg L−1) | Found (μg L−1) | Recovery (%) | texp | Added (μg L−1) | Found (μg L−1) | Recovery (%) | texp | |
| Rum-1 | - | 0.090 ± 0.007 | - | - | - | 6.20 ± 0.90 | - | - | - | 2.12 ± 0.15 | - | - |
| 0.200 | 0.284 ± 0.017 | 97.0 | 0.611 | 0.500 | 6.67 ± 0.50 | 94.0 | 0.104 | 0.500 | 2.60 ± 0.18 | 96.0 | 0.192 | |
| Rum-2 | - | <LOD | - | - | - | 7.90 ± 0.60 | - | - | - | 1.48 ± 0.10 | - | - |
| 0.200 | 0.190 ± 0.014 | 95.0 | 1.237 | 0.500 | 8.41 ± 0.80 | 102.0 | −0.022 | 0.500 | 2.000 ± 0.11 | 104.0 | −0.315 | |
| Vodka-1 | - | <LOD | - | - | - | 10.30 ± 0.90 | - | - | - | <LOD | - | - |
| 0.200 | 0.188 ± 0.012 | 94.0 | 1.732 | 0.500 | 10.78 ± 0.80 | 96.0 | 0.043 | 0.500 | 0.488 ± 0.661 | 97.6 | 0.031 | |
| Vodka-2 | - | <LOD | - | - | - | 4.60 ± 0.30 | - | - | - | 0.220 ± 0.011 | - | - |
| 0.200 | 0.192 ± 0.015 | 96.0 | 0.924 | 0.500 | 5.09 ± 0.30 | 98.0 | 0.058 | 0.500 | 0.700 ± 0.032 | 96.0 | 1.155 | |
| Gin-1 | - | <LOD | - | - | - | 5.90 ± 0.20 | - | - | - | <LOD | - | - |
| 0.200 | 0.210 ± 0.017 | 105.0 | −1.019 | 0.500 | 6.38 ± 0.40 | 96.0 | 0.087 | 0.500 | 0.475 ± 0.033 | 95.0 | 1.443 | |
| Gin-2 | - | 0.120 ± 0.009 | - | - | - | 9.20 ± 0.70 | - | - | - | 1.50 ± 0.12 | - | - |
| 0.200 | 0.325 ± 0.030 | 102.5 | −0.289 | 0.500 | 9.73 ± 0.60 | 106.0 | −0.087 | 0.500 | 1.97 ± 0.11 | 94.0 | 0.472 | |
| Tsipouro-1 | - | 0.100 ± 0.009 | - | - | - | 12.30 ± 1.00 | - | - | - | 30.2 ± 1.6 1 | - | - |
| 0.200 | 0.293 ± 0.020 | 96.5 | 0.606 | 0.500 | 12.83 ± 1.10 | 106.0 | −0.047 | 5.0 | 35.1 ± 1.8 | 98.0 | 0.096 | |
| Tsipouro-2 | - | <LOD | - | - | - | 12.10 ± 0.90 | - | - | - | 25.4 ± 5.0 1 | - | - |
| 0.200 | 0.205 ± 0.015 | 102.5 | −0.577 | 12.59 ±0.80 | 98.0 | 0.022 | 5.0 | 30.5 ± 1.7 | 101.0 | −0.551 | ||
| Sample | Cd | Cu | Pb | Ref. |
|---|---|---|---|---|
| Gin | 0.08–1.12 | - | - | [28] |
| Rum | ND–0.70 | - | - | |
| Rum | 3.0–30 | ND–640 | 50–220 | [29] |
| Gin | 10–30 | ND–70 | 100–130 | |
| Vodka | 10–30 | ND–90 | 80–380 | |
| Rum | - | 3–45 | 23–65 | [30] |
| Fruit spirits | ND–6.6 | - | - | [31] |
| Gin | - | - | ND–35.70 | [32] |
| Rum | - | - | ND–70.00 | |
| Pomace brandy (Tsikoudia) | ND–1 | 55–105,000 | <1–1200 | [33] |
| Rum | ND | ND–276,000 | ND–16 | [34] |
| Rum | - | ND–400 | - | [35] |
| Rum | - | - | 1.25 | [36] |
| Rum, vodka, gin, tsipouro | ND–0.120 | 4.60–12.30 | ND–30.2 | This work |
| Analyte | On-Line Procedure | P.T. (s) | S.C. (mL) | E.F. | RSD (%) | LOD (μg L−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Cd(II)/ Pb(II) | SI-DLLME | 90 | 8.1 | 34/ 80 | 4.1/ 3.8 | 0.002/ 0.01 | [37] |
| Cd(II) | SI-SPE based on octadecylsilane functionalized maghemite particles | 250 | 5.0 | 19 | 3.9 | 0.003 | [38] |
| Cd(II) | SI–bead injection–lab-on-valve platform equipped with a microcolumn packed with PTFE beads | 52 | 1.25 | 17.2 | 4.3 | 0.015 | [39] |
| Cu(II) | SI-SPE based on silk fibroin sorbent | 90 | 0.9 | 27.3 | 2.2 | 0.008 | [40] |
| Cd(II) | SI-single-drop micro-extraction | 600 | 15 | 10 | 3.9 | 0.01 | [41] |
| Pb(II) | FI-SPE based on a chelating resin immobilized on aminopropyl-controlled pore glass | 90 | 3.3 | 20.5 | 3.2 | 0.012 | [42] |
| Cd(II) | SI-solvent extraction-back extraction | 130 | 13.0 | 21.4 | 0.4 | 0.0027 | [43] |
| Cu(II) | SI-SPE based on PTFE-beads-packed column | 17 | 1.0 | 20 | 1.8 | 0.015 | [44] |
| Cd(II)/ Cu(II)/ Pb(II) | SI-FDSE | 90 | 13.5 | 38/ 120/ 85 | 2.4/ 2.2/ 2.9 | 0.0019/ 0.0071/ 0.0173 | This work |
| Step a | V Position | SV Position | SP Flow Rate (μL s−1) | SP Operation | SP Volume (μL) | P Operation | Commentary |
|---|---|---|---|---|---|---|---|
| 1 | IN | 2 | 80 | Aspirate | 50 | OFF (*) | Water into SP |
| 2 | OUT | 2 | 5 | Aspirate | 10 | OFF | Air segment into HC |
| 3 | OUT | 3 | 80 | Aspirate | 910 | OFF | APDC into HC |
| 4 | OUT | 4 | 10 | Dispense | 900 | ON (*) | Sample loading, preconcentration for 90 s |
| 5 | OUT | 1 | 80 | Dispense | 70 | OFF | Emptying the SP |
| 6 | IN | 1 | 80 | Aspirate | 300 | OFF | Water into SP |
| 7 | OUT | 6 | 10 | Aspirate | 500 | OFF | MIBK into HC |
| 8 | OUT | 4 | 5 | Dispense | 280 | OFF | Elution– Transportation of eluent up to the exit of DT |
| 9 | DT into GF | ||||||
| 10 | OUT | 4 | 5 | Dispense | 35 | OFF | 35 μL of MIBK into GF |
| 11 | DT back to waste. Starting the ETAAS program/measuring | ||||||
| 12 | OUT | 4 | 50 | Dispense | 485 | OFF | Cleaning of micro-column, Emptying the SP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Kabir, A.; Furton, K.G.; Anthemidis, A. Sol-Gel Graphene Oxide-Coated Fabric Disks as Sorbents for the Automatic Sequential-Injection Column Preconcentration for Toxic Metal Determination in Distilled Spirit Drinks. Molecules 2023, 28, 2103. https://doi.org/10.3390/molecules28052103
Manousi N, Kabir A, Furton KG, Anthemidis A. Sol-Gel Graphene Oxide-Coated Fabric Disks as Sorbents for the Automatic Sequential-Injection Column Preconcentration for Toxic Metal Determination in Distilled Spirit Drinks. Molecules. 2023; 28(5):2103. https://doi.org/10.3390/molecules28052103
Chicago/Turabian StyleManousi, Natalia, Abuzar Kabir, Kenneth G. Furton, and Aristidis Anthemidis. 2023. "Sol-Gel Graphene Oxide-Coated Fabric Disks as Sorbents for the Automatic Sequential-Injection Column Preconcentration for Toxic Metal Determination in Distilled Spirit Drinks" Molecules 28, no. 5: 2103. https://doi.org/10.3390/molecules28052103
APA StyleManousi, N., Kabir, A., Furton, K. G., & Anthemidis, A. (2023). Sol-Gel Graphene Oxide-Coated Fabric Disks as Sorbents for the Automatic Sequential-Injection Column Preconcentration for Toxic Metal Determination in Distilled Spirit Drinks. Molecules, 28(5), 2103. https://doi.org/10.3390/molecules28052103