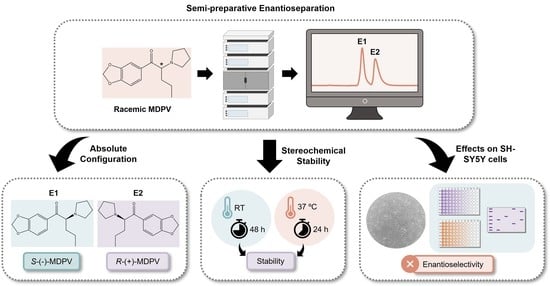
Semi-Preparative Separation, Absolute Configuration, Stereochemical Stability and Effects on Human Neuronal Cells of MDPV Enantiomers
Abstract
:1. Introduction
2. Results
2.1. Semi-Preparative Enantioresolution of MDPV and Evaluation of the Enantiomeric Purity
2.2. Determination of the Absolute Configuration of the Enantiomers of MDPV
2.3. Racemization Study
2.4. Biological/Toxicological Activity of MDPV Enantiomers in SH-SY5Y Cells
2.4.1. Effects in Cytotoxicity
2.4.2. Effects in the Expression of Proteins Involved in Neuroplasticity
3. Discussion
4. Materials and Methods
4.1. Reagents and Samples
4.2. Sample Preparation
4.3. Instrumentation and Chromatographic Conditions
4.3.1. Semi-Preparative Enantioseparation
4.3.2. Enantiomeric Purity Evaluation and Racemization Study
4.4. Chromatographic Parameters Determination
4.5. Determination of the Absolute Configuration of the Enantiomers by ECD
4.6. Cell Culture
4.7. Cytotoxicity Assays
4.7.1. MTT Reduction Assay
4.7.2. Neutral Red Assay
4.8. Preparation of Total Protein Extracts
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zanda, M.T.; Fattore, L. Chapter 29—Novel Psychoactive Substances: A New Behavioral and Mental Health Threat. In Addictive Substances and Neurological Disease; Watson, R.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 341–353. [Google Scholar] [CrossRef]
- Coppola, M.; Mondola, R.; Oliva, F.; Picci, R.L.; Ascheri, D.; Trivelli, F. Chapter 63—Treating the Phenomenon of New Psychoactive Substances: Synthetic Cannabinoids and Synthetic Cathinones. In Neuropathology of Drug Addictions and Substance Misuse; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 679–686. [Google Scholar] [CrossRef]
- Zawilska, J.B. Chapter Thirteen—“Legal Highs”—An Emerging Epidemic of Novel Psychoactive Substances. Int. Rev. Neurobiol. 2015, 120, 273–300. [Google Scholar]
- EMCDDA. New Psychoactive Substances: Global Markets, Glocal Threats and the COVID-19 Pandemic—An Update from the EU Early Warning System. 2020. Available online: https://www.emcdda.europa.eu/publications/rapid-communication/new-psychoactive-substances-global-markets-glocal-threats-and-covid-19-pandemic_en (accessed on 18 March 2021).
- Soares, J.; Costa, V.M.; Bastos, M.d.L.; Carvalho, F.; Capela, J.P. An updated review on synthetic cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- German, C.L.; Fleckenstein, A.E.; Hanson, G.R. Bath salts and synthetic cathinones: An emerging designer drug phenomenon. Life Sci. 2014, 97, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Rothman, R.B.; Goldberg, S.R.; Lupica, C.R.; Sitte, H.H.; et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacol 2013, 38, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Bretteville-Jensen, A.L.; Tuv, S.S.; Bilgrei, O.R.; Fjeld, B.; Bachs, L. Synthetic cannabinoids and cathinones: Prevalence and markets. Forensic Sci. Rev. 2013, 25, 7–26. [Google Scholar]
- Protti, M. Chapter 21—Review of Bath Salts on Illicit Drug Market. In Critical Issues in Alcohol and Drugs of Abuse Testing, 2nd ed.; Dasgupta, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 259–271. [Google Scholar] [CrossRef]
- Paillet-Loilier, M.; Cesbron, A.; Boisselier, R.; Bourgine, J.; Debruyne, D. Emerging drugs of abuse: Current perspectives on substituted cathinones. Subst. Abus. Rehabil. 2014, 5, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Kuropka, P.; Zawadzki, M.; Szpot, P. A review of synthetic cathinones emerging in recent years (2019–2022). Forensic Toxicol. 2022, 41, 25–46. [Google Scholar] [CrossRef]
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in Drug Pharmacokinetics and Toxicity: Pharmacological Relevance and Analytical Methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef]
- Almeida, A.S.; Silva, B.; Pinho, P.G.; Remião, F.; Fernandes, C. Synthetic Cathinones: Recent Developments, Enantioselectivity Studies and Enantioseparation Methods. Molecules 2022, 27, 2057. [Google Scholar] [CrossRef]
- Silva, B.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. Chiral Resolution and Enantioselectivity of Synthetic Cathinones: A Brief Review. J. Anal. Toxicol. 2018, 42, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Francotte, E.R. Enantioselective chromatography as a powerful alternative for the preparation of drug enantiomers. J. Chromatogr. A 2001, 906, 379–397. [Google Scholar] [CrossRef]
- Tiritan, M.E.; Pinto, M.; Fernandes, C. Enantioselective Synthesis, Enantiomeric Separations and Chiral Recognition. Molecules 2020, 25, 1713. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.M.M.; Fernandes, C.; Tiritan, M.E. Chiral Separations in Preparative Scale: A Medicinal Chemistry Point of View. Molecules 2020, 25, 1931. [Google Scholar] [CrossRef]
- Hägele, J.S.; Hubner, E.M.; Schmid, M.G. Chiral separation of cathinone derivatives using β-cyclodextrin-assisted capillary electrophoresis-Comparison of four different β-cyclodextrin derivatives used as chiral selectors. Electrophoresis 2019, 40, 1787–1794. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Alcaraz, A.; Borrull, F.; Aguilar, C.; Calull, M. Enantioselective determination of cathinones in urine by high pressure in-line SPE-CE. Electrophoresis 2019, 40, 1762–1770. [Google Scholar] [CrossRef]
- Aturki, Z.; Schmid, M.G.; Chankvetadze, B.; Fanali, S. Enantiomeric separation of new cathinone derivatives designer drugs by capillary electrochromatography using a chiral stationary phase, based on amylose tris(5-chloro-2-methylphenylcarbamate). Electrophoresis 2014, 35, 3242–3249. [Google Scholar] [CrossRef]
- Albals, D.; Heyden, Y.V.; Schmid, M.G.; Chankvetadze, B.; Mangelings, D. Chiral separations of cathinone and amphetamine-derivatives: Comparative study between capillary electrochromatography, supercritical fluid chromatography and three liquid chromatographic modes. J. Pharm. Biomed. Anal. 2016, 121, 232–243. [Google Scholar] [CrossRef]
- Alremeithi, R.H.; Meetani, M.A.; Khalil, S.A. A validated gas chromatography mass spectrometry method for simultaneous determination of cathinone related drug enantiomers in urine and plasma. RSC Adv. 2016, 6, 80576–80584. [Google Scholar] [CrossRef]
- Alremeithi, R.; Meetani, M.A.; Alaidaros, A.A.; Lanjawi, A.; Alsumaiti, K. Simultaneous Quantitative Determination of Synthetic Cathinone Enantiomers in Urine and Plasma Using GC-NCI-MS. J. Anal. Methods Chem. 2018, 2018, 4396043. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, C.; Lima, R.; Pinto, M.M.M.; Tiritan, M.E. Chromatographic supports for enantioselective liquid chromatography: Evolution and innovative trends. J. Chromatogr. A 2022, 1684, 463555. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Fernandes, C.; Tiritan, M.E.; Pinto, M.M.; Valente, M.J.; Carvalho, M.; de Pinho, P.G.; Remião, F. Chiral enantioresolution of cathinone derivatives present in “legal highs”, and enantioselectivity evaluation on cytotoxicity of 3,4-methylenedioxypyrovalerone (MDPV). Forensic Toxicol. 2016, 34, 372–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, B.; Pereira, J.A.; Cravo, S.; Araújo, A.M.; Fernandes, C.; Pinto, M.M.M.; de Pinho, P.G.; Remião, F. Multi-milligram resolution and determination of absolute configuration of pentedrone and methylone enantiomers. J. Chromatogr. B 2018, 1100–1101, 158–164. [Google Scholar] [CrossRef]
- Kolanos, R.; Partilla, J.S.; Baumann, M.H.; Hutsell, B.A.; Banks, M.L.; Negus, S.S.; Glennon, R.A. Stereoselective Actions of Methylenedioxypyrovalerone (MDPV) To Inhibit Dopamine and Norepinephrine Transporters and Facilitate Intracranial Self-Stimulation in Rats. ACS Chem. Neurosci. 2015, 6, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Gannon, B.M.; Williamson, A.; Suzuki, M.; Rice, K.C.; Fantegrossi, W.E. Stereoselective Effects of Abused “Bath Salt” Constituent 3,4-Methylenedioxypyrovalerone in Mice: Drug Discrimination, Locomotor Activity, and Thermoregulation. J. Pharm. Exp. Ther. 2016, 356, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.S.; Silva, B.; Remião, F.; Fernandes, C. Assessment of the Permeability of 3,4-Methylenedioxypyrovalerone (MDPV) across the Caco-2 Monolayer for Estimation of Intestinal Absorption and Enantioselectivity. Int. J. Mol. Sci. 2023, 24, 2680. [Google Scholar] [CrossRef]
- Silva, B.; Silva, R.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. Enantioselectivity on the absorption of methylone and pentedrone using Caco-2 cell line: Development and validation of an UHPLC method for cathinones quantification. Toxicol. Appl. Pharm. 2020, 395, 114970. [Google Scholar] [CrossRef]
- Silva, B.; Rodrigues, J.S.; Almeida, A.S.; Lima, A.R.; Fernandes, C.; Guedes de Pinho, P.; Miranda, J.P.; Remião, F. Enantioselectivity of Pentedrone and Methylone on Metabolic Profiling in 2D and 3D Human Hepatocyte-like Cells. Pharmaceuticals 2022, 15, 368. [Google Scholar] [CrossRef]
- Castrén, E.; Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Bernhardi, L.E.; Eugenín, J. What Is Neural Plasticity? Adv. Exp. Med. Biol. 2017, 1015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Poo, M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001, 2, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Filip, M.; Faron-Górecka, A.; Kuśmider, M.; Gołda, A.; Frankowska, M.; Dziedzicka-Wasylewska, M. Alterations in BDNF and trkB mRNAs following acute or sensitizing cocaine treatments and withdrawal. Brain Res. 2006, 1071, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, F.; Caffino, L.; Racagni, G.; Riva, M.A. Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Eur. Neuropsychopharmacol. 2009, 19, 402–408. [Google Scholar] [CrossRef]
- Fumagalli, F.; Moro, F.; Caffino, L.; Orrù, A.; Cassina, C.; Giannotti, G.; Di Clemente, A.; Racagni, G.; Riva, M.A.; Cervo, L. Region-specific effects on BDNF expression after contingent or non-contingent cocaine i.v. self-administration in rats. Int. J. Neuropsychopharmacol. 2013, 16, 913–918. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wolf, M.E. Multiple faces of BDNF in cocaine addiction. Behav. Brain Res. 2015, 279, 240–254. [Google Scholar] [CrossRef] [Green Version]
- Caffino, L.; Mottarlini, F.; Bilel, S.; Targa, G.; Tirri, M.; Maggi, C.; Marti, M.; Fumagalli, F. Single Exposure to the Cathinones MDPV and α-PVP Alters Molecular Markers of Neuroplasticity in the Adult Mouse Brain. Int. J. Mol. Sci. 2021, 22, 7397. [Google Scholar] [CrossRef]
- Duart-Castells, L.; López-Arnau, R.; Vizcaíno, S.; Camarasa, J.; Pubill, D.; Escubedo, E. 7,8-Dihydroxyflavone blocks the development of behavioral sensitization to MDPV, but not to cocaine: Differential role of the BDNF-TrkB pathway. Biochem. Pharm. 2019, 163, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Nadal-Gratacós, N.; Alberto-Silva, A.S.; Rodríguez-Soler, M.; Urquizu, E.; Espinosa-Velasco, M.; Jäntsch, K.; Holy, M.; Batllori, X.; Berzosa, X.; Pubill, D.; et al. Structure-Activity Relationship of Novel Second-Generation Synthetic Cathinones: Mechanism of Action, Locomotion, Reward, and Immediate-Early Genes. Front. Pharm. 2021, 12, 749429. [Google Scholar] [CrossRef]
- Cortés, N.; Guzmán-Martínez, L.; Andrade, V.; González, A.; Maccioni, R.B. CDK5: A Unique CDK and Its Multiple Roles in the Nervous System. J. Alzheimers Dis. 2019, 68, 843–855. [Google Scholar] [CrossRef]
- Benavides, D.R.; Bibb, J.A. Role of Cdk5 in drug abuse and plasticity. Ann. N. Y. Acad. Sci. 2004, 1025, 335–344. [Google Scholar] [CrossRef]
- Bibb, J.A.; Chen, J.; Taylor, J.R.; Svenningsson, P.; Nishi, A.; Snyder, G.L.; Yan, Z.; Sagawa, Z.K.; Ouimet, C.C.; Nairn, A.C.; et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 2001, 410, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Grimm, J.W.; Shaham, Y.; Hope, B.T. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J. Neurochem. 2003, 85, 1604–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedzony, K.; Markowicz-Kula, K.; Chocyk, A.; Fijał, K.; Maćkowiak, M. The effect of ‘binge’ cocaine administration on the expression of cyclin-dependent kinase 5 and its activator p35 in various regions of rat brain. Brain Res. 2005, 1063, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Duart-Castells, L.; Blanco-Gandía, M.C.; Ferrer-Pérez, C.; Puster, B.; Pubill, D.; Miñarro, J.; Escubedo, E.; Rodríguez-Arias, M. Cross-reinstatement between 3,4-methylenedioxypyrovalerone (MDPV) and cocaine using conditioned place preference. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109876. [Google Scholar] [CrossRef]
- Duart-Castells, L.; López-Arnau, R.; Buenrostro-Jáuregui, M.; Muñoz-Villegas, P.; Valverde, O.; Camarasa, J.; Pubill, D.; Escubedo, E. Neuroadaptive changes and behavioral effects after a sensitization regime of MDPV. Neuropharmacology 2019, 144, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Matlin, S.A.; Tiritan, M.E.; Crawford, A.J.; Cass, Q.B.; Boyd, D.R. HPLC with carbohydrate carbamate chiral phases: Influence of chiral phase structure on enantioselectivity. Chirality 1994, 6, 135–140. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Valente, M.J.; Bastos, M.d.L.; Fernandes, E.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. Neurotoxicity of β-Keto Amphetamines: Deathly Mechanisms Elicited by Methylone and MDPV in Human Dopaminergic SH-SY5Y Cells. ACS Chem. Neurosci. 2017, 8, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Deschamps, J.R.; Jacobson, A.E.; Rice, K.C. Chiral Resolution and Absolute Configuration of the Enantiomers of the Psychoactive “Designer Drug” 3,4-Methylenedioxypyrovalerone. Chirality 2015, 27, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, C.W.; Thorndike, E.B.; Suzuki, M.; Rice, K.C.; Baumann, M.H. Pharmacological mechanisms underlying the cardiovascular effects of the “bath salt” constituent 3,4-methylenedioxypyrovalerone (MDPV). Br. J. Pharm. 2016, 173, 3492–3501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldubayyan, A.A.; Castrignanò, E.; Elliott, S.; Abbate, V. Development and validation of a chiral LC-MS/MS method for the separation and quantification of four synthetic cathinones in human whole blood and its application in stability analysis. Talanta 2023, 253, 123986. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, D.; Fan, J.; Zhong, Y.; Zhang, M.; He, L.; Zhang, W. HPLC semi-preparative separation of diclazuril enantiomers and racemization in solution. J. Sep. Sci. 2020, 43, 1240–1247. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Davies, N.M.; Teng, X.W. Importance of chirality in drug therapy and pharmacy practice: Implications for psychiatry. Adv. Pharm. 2003, 1, 242–252. [Google Scholar]
- Steber, S.E.; Pham, A.N.D.L.; Nelson, E.; Wolf, C. Enantioseparation and racemization of α-aryl-α-fluoroacetonitriles. Chirality 2021, 33, 891–898. [Google Scholar] [CrossRef]
- Hofmann, J.; Fayez, S.; Scheiner, M.; Hoffmann, M.; Oerter, S.; Appelt-Menzel, A.; Maher, P.; Maurice, T.; Bringmann, G.; Decker, M. Sterubin: Enantioresolution and Configurational Stability, Enantiomeric Purity in Nature, and Neuroprotective Activity In Vitro and In Vivo. Chem. Eur. J. 2020, 26, 7299–7308. [Google Scholar] [CrossRef]
- Anizan, S.; Concheiro, M.; Lehner, K.R.; Bukhari, M.O.; Suzuki, M.; Rice, K.C.; Baumann, M.H.; Huestis, M.A. Linear pharmacokinetics of 3,4-methylenedioxypyrovalerone (MDPV) and its metabolites in the rat: Relationship to pharmacodynamic effects. Addict. Biol. 2016, 21, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Tsujikawa, K.; Mikuma, T.; Kuwayama, K.; Miyaguchi, H.; Kanamori, T.; Iwata, Y.T.; Inoue, H. Degradation pathways of 4-methylmethcathinone in alkaline solution and stability of methcathinone analogs in various pH solutions. Forensic Sci. Int. 2012, 220, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Costa, V.M.; Gaspar, H.; Santos, S.; de Lourdes Bastos, M.; Carvalho, F.; Capela, J.P. Structure-cytotoxicity relationship profile of 13 synthetic cathinones in differentiated human SH-SY5Y neuronal cells. NeuroToxicology 2019, 75, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Amaral, C.; Correia-da-Silva, G.; Duarte, J.A.; Bastos, M.L.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. Methylone and MDPV activate autophagy in human dopaminergic SH-SY5Y cells: A new insight into the context of β-keto amphetamines-related neurotoxicity. Arch. Toxicol. 2017, 91, 3663–3676. [Google Scholar] [CrossRef] [PubMed]
- Strother, L.; Miles, G.B.; Holiday, A.R.; Cheng, Y.; Doherty, G.H. Long-term culture of SH-SY5Y neuroblastoma cells in the absence of neurotrophins: A novel model of neuronal ageing. J. Neurosci. Methods 2021, 362, 109301. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, B.; Palmeira, A.; Silva, R.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. S-(+)-Pentedrone and R-(+)-methylone as the most oxidative and cytotoxic enantiomers to dopaminergic SH-SY5Y cells: Role of MRP1 and P-gp in cathinones enantioselectivity. Toxicol. Appl. Pharm. 2021, 416, 115442. [Google Scholar] [CrossRef] [PubMed]
- Tiritan, M.E.; Fernandes, C.; Maia, A.S.; Pinto, M.; Cass, Q.B. Enantiomeric ratios: Why so many notations? J. Chromatogr. A 2018, 1569, 1–7. [Google Scholar] [CrossRef]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2010, 22, 229–233. [Google Scholar] [CrossRef]
- Silva, J.P.; Carmo, H.; Carvalho, F. The synthetic cannabinoid XLR-11 induces in vitro nephrotoxicity by impairment of endocannabinoid-mediated regulation of mitochondrial function homeostasis and triggering of apoptosis. Toxicol. Lett. 2018, 287, 59–69. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, A.S.; Silva, B.; Silva, J.P.; Pereira, J.A.; Remião, F.; Fernandes, C. Semi-Preparative Separation, Absolute Configuration, Stereochemical Stability and Effects on Human Neuronal Cells of MDPV Enantiomers. Molecules 2023, 28, 2121. https://doi.org/10.3390/molecules28052121
Almeida AS, Silva B, Silva JP, Pereira JA, Remião F, Fernandes C. Semi-Preparative Separation, Absolute Configuration, Stereochemical Stability and Effects on Human Neuronal Cells of MDPV Enantiomers. Molecules. 2023; 28(5):2121. https://doi.org/10.3390/molecules28052121
Chicago/Turabian StyleAlmeida, Ana Sofia, Bárbara Silva, João Pedro Silva, José Augusto Pereira, Fernando Remião, and Carla Fernandes. 2023. "Semi-Preparative Separation, Absolute Configuration, Stereochemical Stability and Effects on Human Neuronal Cells of MDPV Enantiomers" Molecules 28, no. 5: 2121. https://doi.org/10.3390/molecules28052121
APA StyleAlmeida, A. S., Silva, B., Silva, J. P., Pereira, J. A., Remião, F., & Fernandes, C. (2023). Semi-Preparative Separation, Absolute Configuration, Stereochemical Stability and Effects on Human Neuronal Cells of MDPV Enantiomers. Molecules, 28(5), 2121. https://doi.org/10.3390/molecules28052121