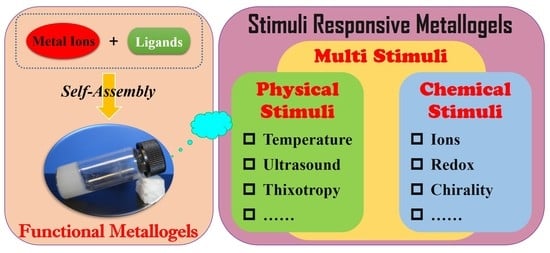
Recent Advances in Stimuli-Responsive Metallogels
Abstract
:1. Introduction
2. Stimuli-Responsive Metallogels
2.1. Chemical Stimuli-Responsive Metallogels
2.1.1. Ion-Responsive Metallogels
2.1.2. Redox-Responsive Metallogels
2.1.3. Chirality-Responsive Metallogels
2.2. Physical Stimuli-Responsive Metallogels
2.2.1. Ultrasound-Responsive Metallogels
2.2.2. Thixotropic Metallogels
2.3. Multi-Stimuli-Responsive Metallogels
2.3.1. Discrete Metal Complex-Based Multi-Stimuli-Responsive Metallogels
2.3.2. Metallopolymer-Based Multi-Stimuli-Responsive Metallogels
2.3.3. Host–Guest Chemistry-Based Multi-Stimuli-Responsive Metallogels
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, R.; Terech, P. Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Weiss, R. Molecular Gels: Structure and Dynamics; The Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- Escuder, B.; Miravet, J.F. Functional Molecular Gels; The Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Terech, P.; Weiss, R.G. Low molecular mass gelators of organic liquids and the properties of their gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Fang, W.; Sun, Z. Visual-size molecular recognition based on gels. Adv. Mater. 2013, 25, 5304–5313. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Schalley, C.A. Exploring macrocycles in functional supramolecular gels: From stimuli responsiveness to systems chemistry. Acc. Chem. Res. 2014, 47, 2222–2233. [Google Scholar] [CrossRef]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-gelators and their applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting-self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jin, Q.X.; Liu, M.H. Enantioselective recognition by chiral supramolecular gels. Chem. Asian J. 2016, 11, 2642–2649. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-molecular-weight gels: The state of the art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Zhang, Y.; Wu, J.; Liu, C.; Zhu, H.; Tu, T. Recent advances in supramolecular gels and catalysis. Chem.—Asian J. 2018, 13, 712–729. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Li, Z.; Ji, X.; Xie, H.; Tang, B.Z. Aggregation-induced emission-active gels: Fabrications, functions, and applications. Adv. Mater. 2021, 33, 2100021. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.L.; Ren, X.Z.; Zhang, J.M.; Lin, Q.; Zhao, Y.L. Supramolecular adhesive hydrogels for tissue engineering applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Steed, J.W. Anion-tuned supramolecular gels: A natural evolution from urea supramolecular chemistry. Chem. Soc. Rev. 2010, 39, 3686–3699. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Zhang, D. Stimuli responsive gels based on low molecular weight gelators. J. Mater. Chem. 2012, 22, 38–50. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Zhang, M.; Yi, T. Low-molecular-mass gels responding to ultrasound and mechanical stress: Towards self-healing materials. Chem. Soc. Rev. 2014, 43, 5346–5371. [Google Scholar] [CrossRef]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [Green Version]
- Draper, E.R.; Adams, D.J. Photoresponsive gelators. Chem. Commun. 2016, 52, 8196–8206. [Google Scholar] [CrossRef] [Green Version]
- Singh, W.P.; Singh, R.S. Gelation-based visual detection of analytes. Soft Mater. 2019, 17, 93–118. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Willner, I. Stimuli-responsive biomolecule-based hydrogels and their applications. Angew. Chem. Int. Ed. 2020, 59, 15342–15377. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Chu, Q.-K.; Feng, Y. Progress in stimulus-responsive dendritic gels. Acta Chim. Sin. 2022, 80, 1424–1435. [Google Scholar] [CrossRef]
- Fages, F. Metal coordination to assist molecular gelation. Angew. Chem. Int. Ed. 2006, 45, 1680–1682. [Google Scholar] [CrossRef] [PubMed]
- Tam, Y.-Y.; Yam, V.W.-W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, J.H.; Silverman, J.R.; John, G. Coordination polymer gels with important environmental and biological applications. Chem. Soc. Rev. 2013, 42, 924–936. [Google Scholar] [CrossRef]
- Zhang, J.; Su, C.-Y. Metal-organic gels: From discrete metallogelators to coordination polymers. Coord. Chem. Rev. 2013, 257, 1373–1408. [Google Scholar] [CrossRef]
- Dastidar, P.; Ganguly, S.; Sarkar, K. Metallogels from coordination complexes, organometallic, and coordination polymers. Chem. Asian J. 2016, 11, 2484–2498. [Google Scholar] [CrossRef]
- Sutar, P.; Maji, T.K. Coordination polymer gels: Soft metal–organic supramolecular materials and versatile applications. Chem. Commun. 2016, 52, 8055–8074. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, J.; Kjøniksen, A.-L.; Wang, W.; Zhang, Y.; Ma, J. Metallogels: Availability, applicability, and advanceability. Adv. Mater. 2019, 31, 1806204. [Google Scholar] [CrossRef]
- Kuosmanen, R.; Rissanen, K.; Sievänen, E. Steroidal supramolecular metallogels. Chem. Soc. Rev. 2020, 49, 1977–1998. [Google Scholar] [CrossRef] [Green Version]
- Sutar, P.; Maji, T.K. Recent advances in coordination-driven polymeric gel materials: Design and applications. Dalton Trans. 2020, 49, 7658–7672. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and anion-binding supramolecular gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Wei, S.-C.; Pan, M.; Li, K.; Wang, S.; Zhang, J.; Su, C.-Y. A multistimuli-responsive photochromic metal-organic gel. Adv. Mater. 2014, 26, 2072–2077. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H.; Lee, M. Stimuli-responsive gels from reversible coordination polymers. Angew. Chem. Int. Ed. 2005, 44, 5810–5814. [Google Scholar] [CrossRef]
- Zhao, G.-Z.; Chen, L.-J.; Wang, W.; Zhang, J.; Yang, G.; Wang, D.-X.; Yu, Y.; Yang, H.-B. Stimuli-responsive supramolecular gels through hierarchical self-assembly of discrete rhomboidal metallacycles. Chem. Eur. J. 2013, 19, 10094–10100. [Google Scholar] [CrossRef]
- Wu, N.-W.; Chen, L.-J.; Wang, C.; Ren, Y.-Y.; Li, X.; Xu, L.; Yang, H.-B. Hierarchical self-assembly of a discrete hexagonal metallacycle into the ordered nanofibers and stimuli-responsive supramolecular gels. Chem. Commun. 2014, 50, 4231–4233. [Google Scholar] [CrossRef]
- Saha, E.; Karthick, K.; Kundu, S.; Mitra, J. Electrocatalytic oxygen evolution in acidic and alkaline media by a multistimuli-responsive Cobalt(II) organogel. ACS Sustain. Chem. Eng. 2019, 7, 16094–16102. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, C.; Wang, G.; Xu, L.; Wang, H. Ag-induced metallogel based on cyclooctatetrathiophene: Structural characterization and stimuli-responsive properties. Soft Matter 2021, 17, 341–345. [Google Scholar] [CrossRef]
- Bera, S.; Chakraborty, A.; Karak, S.; Halder, A.; Chatterjee, S.; Saha, S.; Banerjee, R. Multistimuli-responsive interconvertible low-molecular weight metallohydrogels and the in situ entrapment of CdS quantum dots therein. Chem. Mater. 2018, 30, 4755–4761. [Google Scholar] [CrossRef]
- Wu, D.; Jiang, R.; Luo, L.; He, Z.; You, J. Bromide anion-triggered visible responsive metallogels based on squaramide complexes. Inorg. Chem. Front. 2016, 3, 1597–1603. [Google Scholar] [CrossRef]
- Liu, Y.; Shangguan, L.; Wang, H.; Xia, D.; Shi, B. A supramolecular polymer network gel with stimuli-responsiveness constructed by orthogonal metal ion coordination and pillar [5]arene-based host–guest recognition. Polym. Chem. 2017, 8, 3783–3787. [Google Scholar] [CrossRef]
- Panja, S.; Panja, A.; Ghosh, K. Supramolecular gels in cyanide sensing: A review. Mater. Chem. Front. 2021, 5, 584–602. [Google Scholar] [CrossRef]
- Deepa, G.D.; Damodaran, K.K. Metal complexation induced supramolecular gels for the detection of cyanide in water. Supramol. Chem. 2020, 32, 276–286. [Google Scholar]
- Chua, M.H.; Shah, K.W.; Zhou, H.; Xu, J. Recent advances in aggregation-induced emission chemosensors for anion sensing. Molecules 2019, 24, 2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.-Y.; Hu, J.-H.; Fu, Q.-Q.; Gui, K.; Yao, Y. A novel long-alkyl-chained acylhydrazone-based supramolecular polymer gel for the ultrasensitive detection and separation of multianalytes. Soft Matter 2019, 15, 4187–4191. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, T.-T.; Zhu, X.; Sun, B.; Yang, Q.-P.; Wei, T.-B.; Zhang, Y.-M. A novel supramolecular metallogel-based high-resolution anion sensor array. Chem. Commun. 2015, 51, 1635–1638. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, T.-T.; Zhu, X.; Wei, T.-B.; Li, H.; Zhang, Y.-M. Rationally introduce multi-competitive binding interactions in supramolecular gels: A simple and efficient approach to develop multi-analyte sensor array. Chem. Sci. 2016, 7, 5341–5346. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Sun, B.; Yang, Q.-P.; Fu, Y.-P.; Zhu, X.; Wei, T.-B.; Zhang, Y.-M. Double metal ions competitively control the guest-sensing process: A facile approach to stimuli-responsive supramolecular gels. Chem. Eur. J. 2014, 20, 11457–11462. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Jin, L.; Chen, T.; Yin, B. Coordination-induced gelation of an L-glutamic acid Schiff base derivative: The anion effect and cyanide-specific selectivity. Chem. Commun. 2016, 52, 768–771. [Google Scholar] [CrossRef]
- Sui, X.; Feng, X.; Hempenius, M.A.; Vancso, G.J. Redox active gels: Synthesis, structures and applications. J. Mater. Chem. B 2013, 1, 1658–1672. [Google Scholar] [CrossRef]
- Guan, W.-L.; Adam, K.M.; Qiu, M.; Zhang, Y.-M.; Yao, H.; Wei, T.-B.; Lin, Q. Research progress of redox-responsive supramolecular gel. Supramol. Chem. 2020, 32, 578–596. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Yu, H.; Abdin, Z.; Khan, R.U.; Haroon, M. Ferrocene-based redox-responsive polymer gels: Synthesis, structures and applications. J. Organomet. Chem. 2017, 828, 38–51. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, L.; Liu, F.; Astruc, D.; Gu, H. Supramolecular redox-responsive ferrocene hydrogels and microgels. Coord. Chem. Rev. 2020, 419, 213406. [Google Scholar] [CrossRef]
- Liu, J.; Yan, J.; Yuan, X.; Liu, K.; Peng, J.; Fang, Y. A novel low-molecular-mass gelator with a redox active ferrocenyl group: Tuning gel formation by oxidation. J. Colloid Interface Sci. 2008, 318, 397–404. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Kraatz, H.-B. Small-peptide-based organogel kit: Towards the development of multicomponent self-sorting organogels. Chem. Eur. J. 2013, 19, 15862–15871. [Google Scholar] [CrossRef]
- Afrasiabi, R.; Kraatz, H.-B. Stimuli-responsive supramolecular gelation in ferrocene–peptide conjugates. Chem. Eur. J. 2013, 19, 17296–17300. [Google Scholar] [CrossRef]
- Adhikari, B.; Kraatz, H.-B. Redox-triggered changes in the self-assembly of a ferrocene–peptide conjugate. Chem. Commun. 2014, 50, 5551–5553. [Google Scholar] [CrossRef] [Green Version]
- Falcone, N.; Basak, S.; Dong, B.; Syed, J.; Ferranco, A.; Lough, A.; She, Z.; Kraatz, H.-B. A ferrocene-tryptophan conjugate: The role of the indolic nitrogen in supramolecular assembly. ChemPlusChem 2017, 82, 1282–1289. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Z.; He, Y.; Shen, R.; Deng, L.; Yang, M.; Liang, Y.; Zhang, Y. Ferrocenoyl phenylalanine: A new strategy toward supramolecular hydrogels with multistimuli responsive properties. J. Am. Chem. Soc. 2013, 135, 13379–13386. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host−guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Li, G.; Liu, X.; Wu, S.; Tong, Z. Redox-responsive gel-sol/sol-gel transition in poly(acrylic acid) aqueous solution containing Fe(III) ions switched by light. J. Am. Chem. Soc. 2008, 130, 16166–16167. [Google Scholar] [CrossRef]
- Tie, J.; Liu, H.; Lv, J.; Wang, B.; Mao, Z.; Zhang, L.; Zhong, Y.; Feng, X.; Sui, X.; Xu, H. Multi-responsive, self-healing and adhesive PVA based hydrogels induced by the ultrafast complexation of Fe3+ ions. Soft Matter 2019, 15, 7404–7411. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Fujita, N.; Shinkai, S. A coordination gelator that shows a reversible chromatic change and sol-gel phase-transition behavior upon oxidative/reductive stimuli. J. Am. Chem. Soc. 2004, 126, 8592–8593. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Zhang, L.; Wang, X.; Cao, H.; Jin, Q.; Liu, M. A dual-functional metallogel of amphiphilic copper(II) quinolinol: Redox responsiveness and enantioselectivity. Chem. Eur. J. 2013, 19, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Dutta, S.; Bairi, P.; Pal, T. Redox-responsive Copper(I) metallogel: A metal−organic hybrid sorbent for reductive removal of Chromium(VI) from aqueous solution. Langmuir 2014, 30, 7833–7841. [Google Scholar] [CrossRef]
- Tang, L.; Chen, X.; Wang, L.; Qu, J. Metallo-supramolecular hydrogels based on amphiphilic polymers bearing a hydrophobic schiff base ligand with rapid self-healing and multi-stimuli responsive properties. Polym. Chem. 2017, 8, 4680–4687. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Kuang, Y.; Gao, Y.; Shi, J.; Zhang, X.X.; Xu, B. A redox responsive, fluorescent supramolecular metallohydrogel consists of nanofibers with single-molecule width. J. Am. Chem. Soc. 2013, 135, 5008–5011. [Google Scholar] [CrossRef] [Green Version]
- Gasnier, A.; Royal, G.; Terech, P. Metallo-supramolecular gels based on a multitopic cyclam bis-terpyridine platform. Langmuir 2009, 25, 8751–8762. [Google Scholar] [CrossRef]
- Gasnier, A.; Bucher, C.; Moutet, J.-C.; Royal, G.; Saint-Aman, E.; Terech, P. Redox-Responsive Metallo-Supramolecular Polymers and Gels Containing bis-Terpyridine Appended Cyclam Ligand. Macromol. Symp. 2011, 304, 87–92. [Google Scholar] [CrossRef]
- Wegner, S.V.; Schenk, F.C.; Witzel, S.; Bialas, F.; Spatz, J.P. Cobalt Cross-Linked Redox-Responsive PEG Hydrogels: From Viscoelastic Liquids to Elastic Solids. Macromolecules 2016, 49, 4229–4235. [Google Scholar] [CrossRef]
- Sun, Z.F.; Lv, F.C.; Cao, L.J.; Liu, L.; Zhang, Y.; Lu, Z. Multistimuli-responsive, moldable supramolecular hydrogels cross-linked by ultrafast complexation of metal ions and biopolymers. Angew. Chem. Int. Ed. 2015, 54, 7944–7948. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Chen, S.-Y.; Li, K.; Yu, X.-Q.; Pu, L. Enantioselective gel collapsing: A new means of visual chiral sensing. J. Am. Chem. Soc. 2010, 132, 7297–7299. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, J.; Liu, M.; Liu, Z. A multi-stimuli-responsive metallohydrogel applied in chiral recognition, adsorption of poisonous anions, and construction of various chiral metal–organic frameworks. Chem. Commun. 2019, 55, 14178–14181. [Google Scholar] [CrossRef]
- Tu, T.; Fang, W.; Bao, X.; Li, X.; Dötz, K.H. Visual chiral recognition through enantioselective metallogel collapsing: Synthesis, characterization, and application of platinum–steroid low-molecular-mass gelators. Angew. Chem. Int. Ed. 2011, 50, 6601–6605. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Harnessing mechanochemical effects with ultrasound-induced reactions. Chem. Sci. 2012, 3, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Bardelang, D. Ultrasound induced gelation: A paradigm shift. Soft Matter 2009, 5, 1969–1971. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Molecular self-assembly and patterning induced by sound waves. The case of gelation. Chem. Soc. Rev. 2009, 38, 2684–2697. [Google Scholar] [CrossRef]
- Naota, T.; Koori, H. Molecules that assemble by sound: An application to the instant gelation of stable organic fluids. J. Am. Chem. Soc. 2005, 127, 9324–9325. [Google Scholar] [CrossRef]
- Komiya, N.; Muraoka, T.; Iida, M.; Miyanaga, M.; Takahashi, K.; Naota, T. Ultrasound-induced emission enhancement based on structure-dependent homo- and heterochiral aggregations of chiral binuclear platinum complexes. J. Am. Chem. Soc. 2011, 133, 16054–16061. [Google Scholar] [CrossRef]
- Isozaki, K.; Takaya, H.; Naota, T. Ultrasound-induced gelation of organic fluids with metalated peptides. Angew. Chem. Int. Ed. 2007, 46, 2855–2857. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, S.; Lan, J.; Tang, Y.; Xue, Y.; You, J. Ultrasound-Induced Switching of Sheetlike Coordination Polymer Microparticles to Nanofibers Capable of Gelating Solvents. J. Am. Chem. Soc. 2009, 131, 1689–1691. [Google Scholar] [CrossRef]
- Liu, K.; Meng, L.; Mo, S.; Zhang, M.; Mao, Y.; Cao, X.; Huang, C.; Yi, T. Colour change and luminescence enhancement in a cholesterol-based terpyridyl platinum metallogel via sonication. J. Mater. Chem. C 2013, 1, 1753–1762. [Google Scholar] [CrossRef]
- Pandey, V.K.; Dixit, M.K.; Manneville, S.; Buche, C.; Dubey, M. A multi-stimuli responsive conductive sonometallogel: A mechanistic insight into the role of ultrasound in gelation. J. Mater. Chem. A 2017, 5, 6211–6218. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, S.; Kang, K.; Ren, J.; Yu, X. Self-assembled copper metallogel bearing terpyridine and its application as a catalyst for the click reaction in water. Langmuir 2022, 38, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Yu, X. Alkali metal ion triggered conductive and stimuli-responsive metallogels. Dye. Pigment. 2020, 184, 108863. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, J.H.; Jaworski, J.; Park, S.; Lee, S.S.; Jung, J.H. The influence of ultrasound on porphyrin-based metallogel formation: Efficient control of H- and J-type aggregations. New J. Chem. 2012, 36, 32–35. [Google Scholar] [CrossRef]
- Shirakawa, M.; Fujita, N.; Shinkai, S. A stable single piece of unimolecularly π-stacked porphyrin aggregate in a thixotropic low molecular weight gel: A one-dimensional molecular template for polydiacetylene wiring up to several tens of micrometers in length. J. Am. Chem. Soc. 2005, 127, 4164–4165. [Google Scholar] [CrossRef]
- Beck, J.B.; Rowan, S.J. Multistimuli, multiresponsive metallo-supramolecular polymers. J. Am. Chem. Soc. 2003, 125, 13922–13923. [Google Scholar] [CrossRef]
- Zhao, Y.; Beck, J.B.; Rowan, S.J.; Jamieson, A.M. Rheological behavior of shear-responsive metallo-supramolecular gels. Macromolecules 2004, 37, 3529–3531. [Google Scholar] [CrossRef]
- Samai, S.; Biradha, K. Chemical and mechano responsive metal−organic gels of bis(benzimidazole)-based ligands with Cd(II) and Cu(II) halide salts: Self-sustainability and gas and dye sorptions. Chem. Mater. 2012, 24, 1165–1173. [Google Scholar] [CrossRef]
- Dey, A.; Mandal, S.K.; Biradha, K. Metal–organic gels and coordination networks of pyridine-3,5-bis(1-methyl-benzimidazole-2-yl) and metal halides: Self-sustainability, mechano, chemical responsiveness and gas and dye sorptions. CrystEngComm 2013, 15, 9769–9778. [Google Scholar] [CrossRef]
- McCarney, E.P.; Byrne, J.P.; Twamley, B.; Martínez-Calvo, M.; Ryan, G.; Möbius, M.E.; Gunnlaugsson, T. Self-assembly formation of a healable lanthanide luminescent supramolecular metallogel from 2,6-bis(1,2,3-triazol-4-yl)pyridine (btp) ligands. Chem. Commun. 2015, 51, 14123–14126. [Google Scholar] [CrossRef]
- Fang, W.; Sun, Z.; Tu, T. Novel supramolecular thixotropic metallohydrogels consisting of rare metal−organic nanoparticles: Synthesis, characterization, and mechanism of aggregation. J. Phys. Chem. C 2013, 117, 25185–25194. [Google Scholar] [CrossRef]
- Li, Y.; Lam, E.S.-H.; Tam, A.Y.-Y.; Wong, K.M.-C.; Lam, W.H.; Wu, L.; Yam, V.W.-W. Cholesterol-/estradiol-appended alkynylplatinum(II) complexes as supramolecular gelators: Synthesis, characterization, photophysical and gelation studies. Chem. Eur. J. 2013, 19, 9987–9994. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O.M.; Clarke, N.; Steed, J.W. Shear induced gelation in a copper(II) metallogel: New aspects of ion-tunable rheology and gel-reformation by external chemical stimuli. Soft Matter 2010, 6, 3541–3547. [Google Scholar] [CrossRef]
- Hamilton, T.D.; Bučar, D.-K.; Baltrusaitis, J.; Flanagan, D.R.; Li, Y.; Ghorai, S.; Tivanski, A.V.; MacGillivray, L.R. Thixotropic hydrogel derived from a product of an organic solid-state synthesis: Properties and densities of metal-organic nanoparticles. J. Am. Chem. Soc. 2011, 133, 3365–3371. [Google Scholar] [CrossRef]
- Xue, M.; Lü, Y.; Sun, Q.; Liu, K.; Liu, Z.; Sun, P. Ag(I)-coordinated supramolecular metallogels based on schiff base ligands: Structural characterization and reversible thixotropic property. Cryst. Growth Des. 2015, 15, 5360–5367. [Google Scholar] [CrossRef]
- Chen, J.; Wang, T.; Liu, M. A hydro-metallogel of an amphiphilic L-histidine with ferric ions: Shear-triggered self-healing and shrinkage. Inorg. Chem. Front. 2016, 3, 1559–1565. [Google Scholar] [CrossRef]
- Liu, J.; He, P.; Yan, J.; Fang, X.; Peng, J.; Liu, K.; Fang, Y. An organometallic super-gelator with multiple-stimulus responsive properties. Adv. Mater. 2008, 20, 2508–2511. [Google Scholar] [CrossRef]
- He, P.; Liu, J.; Liu, K.; Ding, L.; Yan, J.; Gao, D.; Fang, Y. Preparation of novel organometallic derivatives of cholesterol and their gel-formation properties. Colloids Surf. A Physicochem. Eng. Asp. 2010, 362, 127–134. [Google Scholar] [CrossRef]
- Yan, J.; Liu, J.; Lei, H.; Kang, Y.; Zhao, C.; Fang, Y. Ferrocene-containing thixotropic molecular gels: Creation and a novel strategy for water purification. J. Colloid Interface Sci. 2015, 448, 374–379. [Google Scholar] [CrossRef]
- He, T.; Li, K.; Wang, N.; Liao, Y.-X.; Wang, X.; Yu, X.-Q. A ferrocene-based multiple-stimuli responsive organometallogel. Soft Matter 2014, 10, 3755–3761. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, R.; Kraatz, H.-B. Rational design and application of a redox-active, photo-responsive, discrete metallogelator. Chem. Eur. J. 2015, 21, 7695–7700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Li, W.; Wu, L. Structural characterization and chemical response of a Ag-coordinated supramolecular gel. Langmuir 2007, 23, 8217–8223. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Chand, D.K. Multi-stimuli-responsive metallogel molded from a Pd2L4-type coordination cage: Selective removal of anionic dyes. Inorg. Chem. 2018, 57, 3634–3645. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Chand, D.K. Nanoscale metallogel via self-assembly of self-assembled trinuclear coordination rings: Multi-stimuli-responsive soft materials. Dalton Trans. 2015, 44, 15181–15188. [Google Scholar] [CrossRef]
- Bunzen, H.; Nonappa; Kalenius, E.; Hietala, S.; Kolehmainen, E. Subcomponent self-assembly: A quick way to new metallogels. Chem. Eur. J. 2013, 19, 12978–12981. [Google Scholar] [CrossRef] [Green Version]
- Jie, K.; Zhou, Y.; Shi, B.; Yao, Y. A Cu2+ specific metallohydrogel: Preparation, multi-responsiveness and pillar [5]arene-induced morphology transformation. Chem. Commun. 2015, 51, 8461–8464. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y.; Tao, X.; Du, G.; Li, Q. Building a quadruple stimuli-responsive supramolecular gel based on a supra-amphiphilic metallogelator. New J. Chem. 2021, 45, 22902–22907. [Google Scholar] [CrossRef]
- Feng, Y.; He, Y.M.; Fan, Q.H. Supramolecular organogels based on dendrons and dendrimers. Chem. Asian J. 2014, 9, 1724–1750. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Z.-X.; Chen, H.; Fan, Q.H. Functional supramolecular gels based on poly(benzyl ether) dendrons and dendrimers. Chem. Commun. 2022, 58, 8736–8753. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Feng, Y.; Zhao, Z.-Y.; Yan, Z.-C.; He, Y.-M.; Luo, X.-J.; Liu, C.-Y.; Fan, Q.-H. A new class of dendritic metallogels with multiple stimuli-responsiveness and as templates for the in situ synthesis of silver nanoparticles. Chem. Eur. J. 2014, 20, 533–541. [Google Scholar] [CrossRef]
- Lakshmi, N.V.; Mandal, D.; Ghosh, S.; Prasad, E. Multi-stimuli-responsive organometallic gels based on ferrocene-linked poly(aryl ether) dendrons: Reversible redox switching and Pb2+-ion sensing. Chem. Eur. J. 2014, 20, 9002–9011. [Google Scholar] [CrossRef]
- Weng, W.; Beck, J.B.; Jamieson, A.M.; Rowan, S.J. Understanding the mechanism of gelation and stimuli-responsive nature of a class of metallo-supramolecular gels. J. Am. Chem. Soc. 2006, 128, 11663–11672. [Google Scholar] [CrossRef]
- Kumpfer, J.R.; Rowan, S.J. Thermo-, photo-, and chemo-responsive shape-memory properties from photo-cross-linked metallo-supramolecular polymers. J. Am. Chem. Soc. 2011, 133, 12866–12874. [Google Scholar] [CrossRef]
- Yuan, J.; Fang, X.; Zhang, L.; Hong, G.; Lin, Y.; Zheng, Q.; Xu, Y.; Ruan, Y.; Weng, W.; Xia, H.; et al. Multi-responsive self-healing metallo-supramolecular gels based on “click” ligand. J. Mater. Chem. 2012, 22, 11515–11522. [Google Scholar] [CrossRef]
- Chen, P.; Li, Q.; Grindy, S.; Holten-Andersen, N. White-light-emitting lanthanide metallogels with tunable luminescence and reversible stimuli-responsive properties. J. Am. Chem. Soc. 2015, 137, 11590–11593. [Google Scholar] [CrossRef]
- Sutar, P.; Maji, T.K. Coordination polymer gels with modular nanomorphologies, tunable emissions, and stimuli-responsive behavior based on an amphiphilic tripodal gelator. Inorg. Chem. 2017, 56, 9417–9425. [Google Scholar] [CrossRef]
- Borré, E.; Stumbé, J.-F.; Bellemin-Laponnaz, S.; Mauro, M. Light-powered self-healable metallosupramolecular soft actuators. Angew. Chem. Int. Ed. 2016, 55, 1313–1317. [Google Scholar] [CrossRef]
- He, Y.; Bian, Z.; Kang, C.; Cheng, Y.; Gao, L. Chiral binaphthylbisbipyridine-based copper(I) coordination polymer gels as supramolecular catalysts. Chem. Commun. 2010, 46, 3532–3534. [Google Scholar] [CrossRef]
- Biswas, P.; Ganguly, S.; Dastidar, P. Stimuli-responsive metallogels for synthesizing Ag nanoparticles and sensing hazardous gases. Chem. Asian J. 2018, 13, 1941–1949. [Google Scholar] [CrossRef]
- Noponen, V.; Toikkanen, K.; Kalenius, E.; Kuosmanen, R.; Salo, H.; Sievänen, E. Stimuli-responsive bile acid-based metallogels forming in aqueous media. Steroids 2015, 97, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiao, D.; Gai, X.; Yan, B.; Ye, H.; Tang, L.; Zhou, Q. A multi-responsive organogel and colloid based on the self-assembly of a Ag(I)-azopyridine coordination polymer. Soft Matter 2021, 17, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhou, Z.; Guan, Z.; Wu, Y.; Liu, Y.; He, J.; Yu, P.; Tu, L.; Zhang, F.; Chen, D.; et al. A multifunctional metallohydrogel with injectability, self-healing, and multistimulus-responsiveness for bioadhesives. Macromol. Mater. Eng. 2018, 303, 1800305. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Ding, X.; Dong, S.; Liu, M.; Zheng, B.; Li, S.; Wu, L.; Yu, Y.; Gibson, H.W.; et al. Metal coordination mediated reversible conversion between linear and cross-linked supramolecular polymers. Angew. Chem. Int. Ed. 2010, 49, 1090–1094. [Google Scholar] [CrossRef]
- Yan, X.; Xu, D.; Chi, X.; Chen, J.; Dong, S.; Ding, X.; Yu, Y.; Huang, F. A multiresponsive, shape-persistent, and elastic supramolecular polymer network gel constructed by orthogonal self-assembly. Adv. Mater. 2012, 24, 362–369. [Google Scholar] [CrossRef]
- Yan, X.; Cook, T.R.; Pollock, J.B.; Wei, P.; Zhang, Y.; Yu, Y.; Huang, F.; Stang, P.J. Responsive supramolecular polymer metallogel constructed by orthogonal coordination-driven self-assembly and host/guest interactions. J. Am. Chem. Soc. 2014, 136, 4460–4463. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, M.; Tang, D.; Yan, X.; Zhang, Z.; Zhou, Z.; Song, B.; Wang, H.; Li, X.; Yin, S.; et al. Fluorescent metallacage-core supramolecular polymer gel formed by orthogonal metal coordination and host−guest interactions. J. Am. Chem. Soc. 2018, 140, 7674–7680. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, D.; Zhang, J.; Ni, R.; Xu, L.; He, T.; Lin, X.; Li, X.; Qiu, H.; Yin, S.; et al. Self-healing heterometallic supramolecular polymers constructed by hierarchical assembly of triply orthogonal interactions with tunable photophysical properties. J. Am. Chem. Soc. 2019, 141, 17909–17917. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Yin, G.; Wang, Y.; Zhang, C.; Shi, J.; Yu, Y.; Yang, H. Cross-linked supramolecular polymer metallogels constructed via self-sorting strategy and their multiple stimuli-responsive behaviors. Chem. Commun. 2015, 51, 16813–16816. [Google Scholar] [CrossRef]
- Li, L.; Cong, Y.; He, L.; Wang, Y.; Wang, J.; Zhang, F.-M.; Bu, W. Multiple stimuli-responsive supramolecular gels constructed from metal–organic cycles. Polym. Chem. 2016, 7, 6288–6292. [Google Scholar] [CrossRef]
- Zhang, Z.-E.; An, Y.-Y.; Zheng, B.; Chang, J.-P.; Han, Y.-F. Hierarchical self-assembly of crown ether based metal-carbene cages into multiple stimuli-responsive cross-linked supramolecular metallogel. Sci. China Chem. 2021, 64, 1177–1183. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Zhang, Y.; Zhang, C.-W.; Chen, L.-J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H.-B. Cross-linked supramolecular polymer gels constructed from discrete multi-pillar [5]arene metallacycles and their multiple stimuli-responsive behavior. J. Am. Chem. Soc. 2014, 136, 8577–8589. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Ou, B.; Jiang, S.-T.; Yin, G.-Q.; Chen, L.-J.; Xu, L.; Li, X.; Yang, H.-B. Cross-linked AIE supramolecular polymer gels with multiple stimuli-responsive behaviours constructed by hierarchical self-assembly. Polym. Chem. 2018, 9, 2021–2030. [Google Scholar] [CrossRef]
- Xia, W.; Ni, M.; Yao, C.; Wang, X.; Chen, D.; Lin, C.; Hu, X.-Y.; Wang, L. Responsive gel-like supramolecular network based on pillar [6]arene−ferrocenium recognition motifs in polymeric matrix. Macromolecules 2015, 48, 4403–4409. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, N.; Xia, W.; Wu, X.; Yao, C.; Liu, X.; Hu, X.-Y.; Lin, C.; Wang, L. Dramatically promoted swelling of a hydrogel by pillar [6]arene-ferrocene complexation with multistimuli responsiveness. J. Am. Chem. Soc. 2016, 138, 6643–6649. [Google Scholar] [CrossRef]
- Lee, Y.H.; He, L.; Chan, Y.-T. Stimuli-responsive supramolecular gels constructed by hierarchical self-assembly based on metal-ligand coordination and host–guest recognition. Macromol. Rapid Commun. 2018, 39, 1800465. [Google Scholar] [CrossRef]
- Yuan, C.; Guo, J.; Tan, M.; Guo, M.; Qiu, L.; Yan, F. Multistimuli responsive and electroactive supramolecular gels based on ionic liquid gemini guest. ACS Macro Lett. 2014, 3, 271–275. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]

























































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhao, X.; Chu, Q.; Feng, Y. Recent Advances in Stimuli-Responsive Metallogels. Molecules 2023, 28, 2274. https://doi.org/10.3390/molecules28052274
Liu Z, Zhao X, Chu Q, Feng Y. Recent Advances in Stimuli-Responsive Metallogels. Molecules. 2023; 28(5):2274. https://doi.org/10.3390/molecules28052274
Chicago/Turabian StyleLiu, Zhixiong, Xiaofang Zhao, Qingkai Chu, and Yu Feng. 2023. "Recent Advances in Stimuli-Responsive Metallogels" Molecules 28, no. 5: 2274. https://doi.org/10.3390/molecules28052274
APA StyleLiu, Z., Zhao, X., Chu, Q., & Feng, Y. (2023). Recent Advances in Stimuli-Responsive Metallogels. Molecules, 28(5), 2274. https://doi.org/10.3390/molecules28052274