Neuroprotective and Antiherpetic Properties of Polyphenolic Compounds from Maackia amurensis Heartwood
Abstract
:1. Introduction
2. Results
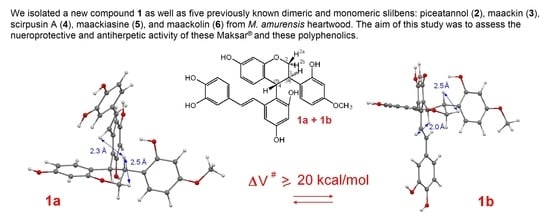
2.1. Structure Determination of Conformers 1a and 1b
2.2. Antiradical Activity and Ferric Reducing Power (FRAP) of Polyphenolic Compounds from M. amurensis Heartwood
2.3. Cytotoxic Activity of Stilbenes from M. amurensis against Neuro-2a Cells
2.4. Effect of Polyphenolic Compounds from M. amurensis Heartwood on the Viability and ROS Level in PQ-Treated Neuro-2a
2.5. Effect of Polyphenolic Compounds from M. amurensis Heartwood on Mitochondrial Membrane Potential in PQ-Treated Neuro-2a
2.6. Cytotoxic Activity of Stilbenes from M. amurensis against Vero Cells
2.7. Anti-HSV-1 Activity of Polyphenolic Compounds from M. amurensis (CPE assay)
2.8. Anti-HSV-1 Activity of Polyphenolic Compounds from M. amurensis (RT-PCR)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. General Experimental Procedures
4.4. HPLC Analysis
4.5. HR-ESI-MS Analysis
4.6. Antiradical Activity
- A0 is the absorbance of DPPH solution without polyphenolic compounds (blank sample);
- Ax is the absorbance of DPPH solution in the presence of different concentrations of polyphenolic compounds.
4.7. Ferric Reducing Antioxidant Power (FRAP) Assay
- CFe is the concentration of Fe2+ (µM) formed in the reaction;
- Cx is the concentration of polyphenolic compounds in the reacting mixture.
4.8. Neuro-2a Cell Line and Culture Conditions
4.9. The Viability of Neuro-2a Cells
4.10. In Vitro Model of PQ-Induced Neurotoxicity
4.11. Reactive Oxygen Species (ROS) Analysis in PQ-Treated Cells
4.12. Mitochondrial Membrane Potential (MMP) Evaluation
4.13. HSV-1 Virus and Vero Cell Culture
4.14. Cytotoxicity of the Tested Compounds against Vero Cells
4.15. Anti-HSV-1 Activity of Stilbenes
- Atv is the absorbance of cells infected with virus and treated with a polyphenolic compound;
- Acv is the absorbance of the untreated virus-infected cells;
- Acd is the absorbance of control (untreated and non-infected) cells.
4.16. Extraction of HSV-1 DNA from Infected Vero Cells
4.17. DNA HSV Detection by the Real-Time Polymerase Chain Reaction (PCR) Method
- Cttv is the average Ct value for the infected samples after treatment with polyphenolic compounds;
- Ctcv corresponds to the average Ct value for the virus control.
- Cttv is the average Ct value for the infected samples after treatment with polyphenolic compound;
- Ctcv corresponds to the average Ct value for the virus control;
- Ctcc corresponds to the average Ct value for the cell control.
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fedoreev, S.A.; Kulish, N.I.; Glebko, L.I.; Pokushalova, T.V.; Veselova, M.V.; Saratikov, A.S.; Vengerovskii, A.I.; Chuchalin, V.S. Maksar: A preparation based on Amur Maackia. Pharm. Chem. J. 2004, 38, 605–610. [Google Scholar] [CrossRef]
- Veselova, M.V.; Fedoreyev, S.A.; Tarbeeva, D.V.; Kulesh, N.I.; Kalinovskiy, A.I.; Kuzmich, A.S.; Kim, N.Y.; Grigorchuk, V.P. Cytotoxic prenylated polyphenolic compounds from Maackia amurensis root bark. Nat. Prod. Commun. 2017, 12, 1037–1040. [Google Scholar] [CrossRef] [Green Version]
- Kulesh, N.I.; Fedoreyev, S.A.; Veselova, M.V.; Mischenko, N.P.; Denisenko, V.A.; Dmitrenok, P.S.; Zverev, Y.F.; Zamyatina, S.V. Antioxidant activity of the isoflavonoids from the roots of Maackia amurensis. Nat. Prod. Commun. 2013, 8, 589–592. [Google Scholar] [CrossRef] [Green Version]
- Iankova, V.I.; Ivanova, I.L.; Fedoreev, S.A.; Kulesh, N.I. Antioxidant activity of the hepatoprotector maxar in experimental diabetes. Eksp. Klin. Farmacol. 2002, 65, 33–36. [Google Scholar]
- Saratikov, A.S.; Chuchalin, V.S.; Ratkin, A.V.; Ratkin, E.V.; Fedoreyev, S.A.; Bulgakov, V.P. Hepatoprotective properties of polyphenolic complexes from wood and cellular culture of maakia amurensis. Bull. Sib. Med. 2008, 7, 51–55. [Google Scholar] [CrossRef]
- Yankova, V.I.; Gvozdenko, T.A.; Ivanova, I.L.; Fedoreev, S.A.; Kulesh, N.I. Effect of Maksar on antioxidant system in rats with type IIa alimentary hyperlipoproteinemia. Bull. Exp. Biol. Med. 2002, 134, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, E.I.; Vengerovsky, A.L.; Gajsaev, R.O.; Saratikov, A.S.; Fedoreev, S.A. The new hepatoprotective agent-maxar. Sib. J. Gastroenterol. Hepatol. 1999, 8, 46–48. [Google Scholar]
- Plotnikova, A.M.; Shulgau, Z.T.; Plotnikova, T.M.; Aliev, O.I.; Kulesh, N.I.; Mischenko, N.P.; Fedoreyev, S.A. Antithrombogenic and antiplatelet activities of extract from Maackia amyrensis wood. Bull. Exp. Biol. Med. 2009, 147, 204–207. [Google Scholar] [CrossRef]
- Fedoreev, S.A.; Kulesh, N.I.; Mishchenko, N.P.; Ermakova, S.P.; Zvjagintseva, T.N. Anticancer Drug. RU Patent 2414920 C1, 1 December 2009. [Google Scholar]
- Utkina, N.K.; Kulesh, N.I. Antioxidant activity of polyphenols and polyphenol complex from the far-eastern tree Maackia amurensis. Pharm. Chem. J. 2012, 46, 488–491. [Google Scholar] [CrossRef]
- Azarova, O.V.; Bryukhanov, V.M.; Bulgakov, V.P.; Zverev, Y.F.; Lampatov, V.V.; Fedoreyev, S.A. Comparative assessment of antioxidant properties of polyphenols from heartwood and Maackia amurensis callus culture. Bull. Sib. Med. 2010, 9, 17–20. [Google Scholar] [CrossRef]
- Ramsay, R.R. Monoamine oxidases: The biochemistry of the proteins as targets in medicinal chemistry and drug discovery. Curr. Top. Med. Chem. 2012, 12, 2189–2209. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, V.P.; Kumar, V. A perspective on monoamine oxidase enzyme as drug target: Challenges and opportunities. Curr. Drug Targets 2017, 18, 87–97. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.J.; Kim, W.J.; Kang, M.G.; Baek, S.C.; Lee, J.P.; Park, D.; Oh, S.R.; Kim, H. Calycosin and 8-O-methylretusin isolated from Maackia amurensis as potent and selective reversible inhibitors of human monoamine oxidase-B. Int. J. Biol. Macromol. 2020, 151, 441–448. [Google Scholar] [CrossRef]
- Lee, H.W.; Ryu, H.W.; Kang, M.G.; Park, D.; Oh, S.R.; Kim, H. Potent selective monoamine oxidase B inhibition by maackiain, a pterocarpan from the roots of Sophora flavescens. Bioorg. Chem. Med. Lett. 2016, 26, 4714–4719. [Google Scholar] [CrossRef]
- Tsai, R.T.; Tsai, C.W.; Liu, S.P.; Gao, J.X.; Kuo, Y.H.; Chao, P.M.; Hung, H.S.; Shyu, W.C.; Lin, S.Z.; Fu, R.H. Maackiain ameliorates 6-hydroxydopamine and SNCA pathologies by modulating the PINK1/Parkin pathway in models of Parkinson’s Disease in Caenorhabditis elegans and the SH-SY5Y cell line. Int. J. Mol. Sci. 2020, 21, 4455. [Google Scholar] [CrossRef]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s Disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limongi, D.; Baldelli, S. Redox imbalance and viral infections in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2016, 2016, 6547248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristen, H.; Santana, S.; Sastre, I.; Recuero, M.; Bullido, M.; Aldudo, J. Herpes simplex virus type 2 infection induces AD-like neurodegeneration markers in human neuroblastoma cells. Neurobiol. Aging 2015, 36, 2737–2747. [Google Scholar] [CrossRef] [Green Version]
- Kavouras, J.H.; Prandovszky, E.; Valyi-Nagy, K.; Kovacs, S.K.; Tiwari, V.; Kovacs, M.; Shukla, D.; Valyi-Nagy, T. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J. Neurovirol. 2007, 13, 416–425. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, G.; Marcocci, M.E.; Sgarbanti, R.; Civitelli, L.; Ripoli, C.; Piacentini, R.; Garaci, E.; Grassi, C.; Palamara, A.T. Infectious agents and neurodegeneration. Mol. Neurobiol. 2012, 46, 614–638. [Google Scholar] [CrossRef] [Green Version]
- Kramer, T.; Enquist, L.W. Alphaherpesvirus infection disrupts mitochondrial transport in neurons. Cell Host Microbe 2012, 11, 504–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, T.; Goshima, F.; Daikoku, T.; Inagaki-Ohara, K.; Takakuwa, H.; Kato, K.; Nishiyama, Y. Mitochondrial distribution and function in herpes simplex virus-infected cells. J. Gen. Virol. 2000, 81, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Santana, S.; Sastre, I.; Recuero, M.; Bullido, M.J.; Aldudo, J. Oxidative stress enhance neurodegeneration markers induced by herpes simplex virus type 1 infection in human neuroblastoma cells. PLoS ONE 2013, 8, e75842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cymerys, J.; Chodkowski, M.; Slonska, A.; Krzyzowska, M.; Banbura, M.W. Disturbances of mitochondrial dynamics in cultured neurons infected with human herpesvirus type 1 and type 2. J. Neurovirol. 2019, 25, 765–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Area-Gomez, E.; de Groof, A.; Bonilla, E.; Montesinos, J.; Tanji, K.; Boldogh, I.; Pon, L.; Schon, E.A. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 2018, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Grunewald, A.; Kumar, K.R.; Sue, C.M. New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol. 2019, 177, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: An overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef] [Green Version]
- Mattio, L.M.; Catinella, G.; Pinto, A.; Dallavalle, S. Natural and nature-inspired stilbenoids as antiviral agents. Eur. J. Med. Chem. 2020, 202, 112541. [Google Scholar] [CrossRef]
- De Clercq, E. Antiviral drugs in current clinical use. J. Clin. Virol. 2004, 30, 115e133. [Google Scholar] [CrossRef]
- Chuanasa, T.; Phromjai, J.; Lipipun, V.; Likhitwitayawuid, K.; Suzuki, M.; Pramyothin, P.; Hattori, M.; Shiraki, K. Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: Mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antivir. Res. 2008, 80, 62e70. [Google Scholar] [CrossRef]
- Kulesh, N.I.; Maksimov, O.B.; Fedoreev, S.A.; Denisenko, V.A.; Glasunov, V.P.; Pokushalova, T.V.; Glebko, L.I. About native components of extracts from Maackia amurensis wood. Chem. Nat. Compd. 1999, 35, 575–579. [Google Scholar] [CrossRef]
- Kulesh, N.I.; Denisenko, V.A.; Maksimov, O.V. Stilbenolignan from Maackia amurensis. Phytochemistry 1995, 40, 1001–1003. [Google Scholar] [CrossRef]
- Krivoshchekova, O.E.; Stepanenko, L.S.; Maksimov, O.B. New isoflavone-stilbene from Maackia amurensis heartwood. Chem. Nat. Cmpd. 1986, 1, 39–42. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ma, F.; Shen, W.; Zhang, X.; Li, M.; Wang, Y.; Zou, Y.; Li, Y.; Wang, H. Anti-HSV activity of Kuwanon X from mulberry leaves with genes expression inhibitory and HSV-1 induced NF-κB deactivated properties. Biol. Pharm. Bull. 2016, 39, 1667–1674. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Qiao, H.; Liu, T.; Yang, Z.; Xu, L.; Xu, Y.; Ge, H.M.; Tan, R.X.; Li, E. Inhibition of herpes simplex virus infection by oligomeric stilbenoids through ROS generation. Antivir. Res. 2012, 95, 30–36. [Google Scholar] [CrossRef]
- Ito, T.; Hayashi, K.; Nishiguchi, M.; Hayashi, T.; Iinuma, M. Resveratrol oligomer C-glucosides and anti-viral resveratrol tetramers isolated from the stem bark of Shorea uliginosa. Phytochem. Lett. 2018, 28, 1–7. [Google Scholar] [CrossRef]
- Mishchenko, N.P.; Krylova, N.V.; Iunikhina, O.V.; Vasileva, E.A.; Likhatskaya, G.N.; Pislyagin, E.A.; Tarbeeva, D.V.; Dmitrenok, P.S.; Fedoreyev, S.A. Antiviral Potential of Sea Urchin Aminated Spinochromes against Herpes Simplex Virus Type 1. Mar. Drugs 2020, 18, 550. [Google Scholar] [CrossRef]
- Tarbeeva, D.V.; Fedoreyev, S.A.; Veselova, M.V.; Blagodatski, A.S.; Klimenko, A.M.; Kalinovskiy, A.I.; Grigorchuk, V.P.; Berdyshev, D.V.; Gorovoy, P.G. Cytotoxic polyphenolic compounds from Lespedeza bicolor stem bark. Fitoterapia 2019, 135, 64–72. [Google Scholar] [CrossRef]
- Dehghan, G.; Khoshkam, Z. Tin(II)-quercetin complex: Synthesis, spectral characterization and antioxidant activity. Food Chem. 2012, 131, 422–426. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Jang, M.J.; Kwon, M.J.; Ahn, Y.J. Antiviral activity and possible mechanism of action of constituents identified in Paeonia lactiflora root toward human rhinoviruses. PLoS ONE 2015, 10, e0121629. [Google Scholar] [CrossRef] [PubMed]
- Weislow, O.S.; Kiser, R.; Fine, D.L.; Bader, J.; Shoemaker, R.H.; Boyd, M.R. New Soluble-Formazan Assay for HIV-1 Cytopathic Effects: Application to High-Flux Screening of Synthetic and Natural Products for AIDS-Antiviral Activity. J. Natl. Cancer Inst. 1989, 81, 577–586. [Google Scholar] [CrossRef] [PubMed]





| Position | 13C | 1H | HMBC | COSY | ROESY |
|---|---|---|---|---|---|
| 2a 2b | 71.6 | 4.24, dd, J = 10.3, 3.5 1H | C-3, 4, 9, 1′ | H-2b, 3 | H-2b, 3 |
| 4.03, t, J = 10.3, 1H | C-3, 4 | H-2a, 3 | H-2a, 4 | ||
| 3 | 39.0 | 4.16, td, J = 11.6, 3.5 1H | C-2, 4, 1′, 2′, 6′ (weak), 1″ | H-2, 4 | H-7″, 2a |
| 4 | 35.8 | 5.36, d, J = 11.6, 1H | C-2 (weak), 3, 5 (weak), 9 (weak), 1″, 2″, 6″ | H-3 | H-2b, H-6′ |
| 5 | 130.1 | 6.64, d, J = 8.4, 1H | C-4, 7, 9 | H-6 | H-6 |
| 6 | 109.0 | 6.29 dd, J = 8.4, 2.4 1H | C-8, 10 | H-5, 8 | H-5 |
| 7 | 156.8 | ||||
| 8 | 103.2 | 6.42, d, J = 2.4, 1H | C-6, 7, 9, 10 | H-6 | |
| 9 | 156.4 | ||||
| 10 | 119.5 | ||||
| 1′ | 119.0 | ||||
| 2′ | 156.8 | ||||
| 3′ | 101.8 | 6.30, d, J = 2.4, 1H | C-1′, 2′, 4′, 5′, | H-5′ | OCH3-4′ |
| 4′ | 159.8 | ||||
| 5′ | 104.9 | 6,27, dd, J = 8.5, 2.4 1H | C-1′, 3′, 4′ | H-3′, 6′ | OCH3-4′ |
| 6′ | 129.8 | 7.25, d, J = 8.5, 1H | C-3, 2′, 4′ | H-5′ | H-4 |
| 1″ | 119.5 | ||||
| 2″ | 157.4 | ||||
| 3″ | 101.7 | 6.26, d, J = 2.5, 1H | C-1″, 2″, 4″, 5″ | H-5″ | |
| 4″ | 156.6 | ||||
| 5″ | 106.0 | 6.51, d, J = 2.5, 1H | C-1″, 3″, 4″, 7″ | H-3″ | |
| 6″ | 139.6 | ||||
| 7″ | 126.2 | 6.82, d, J = 16.0, 1H | C-1″, 5″, 6″ (weak), 9″ | H-8″ | H-3 |
| 8″ | 129.0 | 6.57, d, J= 16.0, 1H | C-6″, 9″, 10″, 14″ | H-7″ | |
| 9″ | 131.2 | ||||
| 10″ | 114.0 | 6.80, d, J = 2.4, 1H | C-8″, 11″, 12″, 14″ | H-14″ | |
| 11″ | 145.6 | ||||
| 12″ | 145.4 | ||||
| 13″ | 115.7 | 6.73, d, J = 8.1, 1H | C-9″, 11″, 12″ | H-14″ | |
| 14″ | 119.2 | 6.67, dd, J = 8.1, 2.4 1H | C-8″, 10″, 12″ | H-10″, 13″ | |
| OCH3-4′ | 54.9 | 3.63, s, 3H | C-4′ | H-3′, 5′ |
| Position | 13C | 1H | HMBC | COSY | ROESY |
|---|---|---|---|---|---|
| 2a 2b | 70.6 | 4.20, dd, J = 10.3, 3.2 1H | C-3, 4, 9, 1′ | H-2b, 3 | H-2b, 3 |
| 4.44, t, J = 10.3, 1H | C-4 (weak) | H-2a, 3 (weak) | H-2a | ||
| 3 | 38.4 | 4.36, td, J = 10.9, 3.2 1H | C-2 (weak), 2′ (weak), 6′ (weak), 1″ (weak) | H-2a, 2b(weak), 4 | H-2a |
| 4 | 39.7 | 4.95, d, J = 10.9, 1H | C-3, 5 (weak), 1″, 2″, 6″ | H-3 | H-7″ |
| 5 | 129.3 | 6.50, d, J = 8.8, 1H | C-4, 7, 9 | H-6 | H-6 |
| 6 | 108.3 | 6.20 dd, J = 8.8, 2.4 1H | C-8, 10, | H-5, 8 | H-5 |
| 7 | 156.6 | ||||
| 8 | 103.1 | 6.27, d, J = 2.4, 1H | C-6, 10 | H-6 | |
| 9 | 156.3 | ||||
| 10 | 119.5 | ||||
| 1′ | 119.7 | ||||
| 2′ | 157.1 | ||||
| 3′ | 102.2 | 6.40, d, J = 2.4, 1H | C-1′, 2′, 4′, 5′ | H-5′ | OCH3-4′ |
| 4′ | 159.9 | ||||
| 5′ | 105.0 | 6,22, dd, J = 8.5, 2.4 1H | C-1′, 3′, 4′ | H-3′, 6′ | H-6′, OCH3-4′ |
| 6′ | 131.1 | 6.99, d, J = 8.5, 1H | C-3, 2′, 4′ | H-5′ | H-5 |
| 1″ | 119.4 | ||||
| 2″ | 157.1 | ||||
| 3″ | 103.6 | 6.20, d, J = 2.4, 1H | C-1″, 2″, 4″, 5″ | H-5″ | |
| 4″ | 156.8 | ||||
| 5″ | 104.8 | 6.44, d, J = 2.4, 1H | C-1″, 3″, 4″, 7″ | H-3″ | |
| 6″ | 141.7 | ||||
| 7″ | 126.1 | 7.25, d, J = 15.9, 1H | C-1″, 5″, 9″ | H-8″ | H-4 |
| 8″ | 131.4 | 6.62, d, J = 15.9, 1H | C-6″, 7″(weak), 9″, 10″, 14″ | H-7″ | H-10″ |
| 9″ | 131.0 | ||||
| 10″ | 113.3 | 7.09, d, J = 1.9, 1H | C-8″, 11″, 12″, 14″ | H-14″ | H-8″ |
| 11″ | 145.8 | ||||
| 12″ | 145.6 | ||||
| 13″ | 115.7 | 6.78, d, J = 8.1, 1H | C-9″, 11″, 12″ | H-14″ | |
| 14″ | 119.9 | 6.84, dd, J = 8.5, 1.9 1H | C-8″, 10″, 12″ | H-10″, 13″ | |
| OCH3-4′ | 54.9 | 3.61, s, 3H | C-4′ | H-3′, 5′ |
| Compound | DPPH Scavenging Effect | FRAP | ||
|---|---|---|---|---|
| IC50 µM, 20 min | IC50 µg, 20 min | CFe2+(µM)/Cpolyphenolic compound (µM) | CFe2+(µM)/Cpolyphenolic compound (μkg/mL) | |
| Quercetin | 9.3 ± 0.4 | 2.81 ± 0.12 | 5.53 ± 0.55 | 18.3 ± 1.82 |
| Ascorbic acid | 33.1 ± 2.8 | 5.83 ± 0.49 | 3.58 ± 0.29 | 20.34 ± 0.16 |
| 1 | 4.3 ± 0.5 * | 2.25 ± 0.25 | 12.36 ± 1.38 ** | 24.04 ± 2.68 * |
| 2 | 4.3 ± 0.5 * | 1.05 ± 0.12 ** | 15.71 ± 1.43 *** | 64.38 ± 5.86 *** |
| 3 | 2.7 ± 0.3 ** | 1.31 ± 0.15 ** | 10.11 ± 0.93 ** | 20.80 ± 1.91 |
| 4 | 3.2 ± 0.4 ** | 1.50 ± 0.19 * | 11.44 ± 1.41 ** | 24.34 ± 3.00 * |
| 5 | 2.5 ± 0.3 ** | 1.32 ± 0.16 ** | 2.50 ± 0.38 | 4.75 ± 0.72 |
| 6 | 2.0 ± 0.3 *** | 0.90 ± 0.14 *** | 23.10 ± 2.65 *** | 51.11 ± 5.86 *** |
| Maksar® | - | 9.9 ± 0.94 * | - | 3.5 ± 0.60* |
| Compounds | CC50 | IC50 | SI | ||
|---|---|---|---|---|---|
| µg/mL | µM | µg/mL | µM | ||
| Maksar® | 1211.7 ± 133 | 13.9 ± 1.8 | 87.2 ± 11.3 | ||
| 1 | 162.4 ± 17.9 | 315.9 ± 34.7 | 14.0 ± 1.5 | 27.2 ± 3.0 | 11.6 ± 1.3 |
| 2 | 250.2 ± 30.0 | 1025.4 ± 123.0 | 22.0 ± 2.6 | 90.2 ± 10.8 | 11.4 ± 1.4 |
| 3 | 269.6 ± 35.0 | 554.7 ± 72.1 | 17.6 ± 2.3 | 36.2 ± 4.7 | 15.3 ± 1.9 |
| 4 | 199.4 ± 23.9 | 424.2 ± 50.9 | 13.8 ± 1.6 | 29.4 ± 3.5 | 14.4 ± 1.7 |
| 5 | 156.4 ± 18.8 | 297.3 ± 35.7 | 23.7 ± 2.8 | 45.0 ± 5.4 | 6.6 ± 0.8 |
| 6 | 256.4 ± 33.3 | 567.2 ± 73.7 | 40.2 ± 5.2 | 88.9 ± 11.5 | 6.4 ± 0.8 |
| Acyclovir | >1000 | >4000 | 2.1 ± 0.3 | 9.3 ± 1.3 | 430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarbeeva, D.V.; Berdyshev, D.V.; Pislyagin, E.A.; Menchinskaya, E.S.; Kim, N.Y.; Kalinovskiy, A.I.; Krylova, N.V.; Iunikhina, O.V.; Persiyanova, E.V.; Shchelkanov, M.Y.; et al. Neuroprotective and Antiherpetic Properties of Polyphenolic Compounds from Maackia amurensis Heartwood. Molecules 2023, 28, 2593. https://doi.org/10.3390/molecules28062593
Tarbeeva DV, Berdyshev DV, Pislyagin EA, Menchinskaya ES, Kim NY, Kalinovskiy AI, Krylova NV, Iunikhina OV, Persiyanova EV, Shchelkanov MY, et al. Neuroprotective and Antiherpetic Properties of Polyphenolic Compounds from Maackia amurensis Heartwood. Molecules. 2023; 28(6):2593. https://doi.org/10.3390/molecules28062593
Chicago/Turabian StyleTarbeeva, Darya V., Dmitry V. Berdyshev, Evgeny A. Pislyagin, Ekaterina S. Menchinskaya, Natalya Y. Kim, Anatoliy I. Kalinovskiy, Natalya V. Krylova, Olga V. Iunikhina, Elena V. Persiyanova, Mikhail Y. Shchelkanov, and et al. 2023. "Neuroprotective and Antiherpetic Properties of Polyphenolic Compounds from Maackia amurensis Heartwood" Molecules 28, no. 6: 2593. https://doi.org/10.3390/molecules28062593
APA StyleTarbeeva, D. V., Berdyshev, D. V., Pislyagin, E. A., Menchinskaya, E. S., Kim, N. Y., Kalinovskiy, A. I., Krylova, N. V., Iunikhina, O. V., Persiyanova, E. V., Shchelkanov, M. Y., Grigorchuk, V. P., Aminin, D. L., & Fedoreyev, S. A. (2023). Neuroprotective and Antiherpetic Properties of Polyphenolic Compounds from Maackia amurensis Heartwood. Molecules, 28(6), 2593. https://doi.org/10.3390/molecules28062593