Magnetostructural Properties of Some Doubly-Bridged Phenoxido Copper(II) Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Ligands and Complexes
2.2. Characterization of the Ligands
2.3. Characterization of the Complexes
2.4. Description of the Structures of Complexes
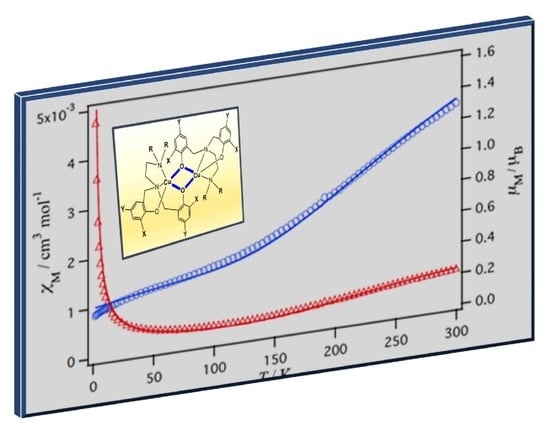
2.5. Magnetic Properties
3. Conclusions
4. Experimental
4.1. Materials and Physical Measurements
4.2. Synthesis of the Organic Ligands
4.2.1. 6,6′-(((2-(Dimethylamino)ethyl)azanediyl)bis(methylene))bis(2-bromo-4-methylphenol) (H2L3)
4.2.2. 6,6′-(((2-(Diethylamino)ethyl)azanediyl)bis(methylene))bis(2,4-dimethylphenol) (H2L4)
4.2.3. 6,6′-(((2-(Diethylamino)ethyl)azanediyl)bis(methylene))bis(4-chloro-2-methylphenol) (H2L9)
4.3. Synthesis of Copper(II) Complexes
4.3.1. [Cu2(µ2-L3)2] (3)
4.3.2. [Cu2(µ2-L4)2]·CH3COCH3 (4)
4.3.3. [Cu3(µ2-L9)2(µ-OCH3)2]·CH3OH (9)
4.4. X-ray Crystal Structure Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Massoud, S.S.; Spell, M.; Ledet, C.; Junk, T.; Herchel, R.; Fischer, R.C.; Travnicek, Z.; Mautner, F.A. Magnetic and structural properties of dinuclear singly bridged-phenoxido metal(II) complexes. Dalton Trans. 2015, 44, 2110–2121. [Google Scholar] [CrossRef]
- Massoud, S.S.; Junk, T.; Louka, F.R.; Herchel, R.; Travnicek, Z.; Fischer, R.C.; Mautner, F.A. Synthesis, structure and magnetic characterization of dinuclear copper(II) complexes bridged by bicompartmental phenolate. RSC Adv. 2015, 5, 87139–87150. [Google Scholar] [CrossRef]
- Massoud, S.S.; Junk, J.; Herchel, R.; Travnicek, Z.; Mikuriya, M.; Fischer, R.C.; Mautner, F.A. Structural characterization of ferromagnetic bridged-acetato and -dichlorido copper(II) complexes based on bicompartmental 4-t-butylphenol. Inorg. Chem. Commun. 2015, 60, 1–3. [Google Scholar] [CrossRef]
- Massoud, S.S.; Ledet, C.C.; Junk, T.; Bosch, S.; Comba, P.; Herchel, R.; Hošek, J.; Trávníček, Z.; Fischer, R.C.; Mautner, F.A. Dinuclear metal(II)-acetato complexes based on bicompartmental chlorophenol: Synthesis, structure, magnetic properties, DNA interaction and phosphodiester hydrolysis. Dalton Trans. 2016, 45, 12933–12950. [Google Scholar] [CrossRef] [PubMed]
- Mikuriya, M.; Sato, S.; Yoshioka, D. µ-Phenolato-µ-acetato-bridged dinuclear copper(II) complex with dinucleating Schiff-base ligand having three phenolate groups. X-Ray Struct. Anal. Online 2018, 34, 45–47. [Google Scholar] [CrossRef]
- Mikuriya, M.; Sato, S.; Yoshioka, D. µ-Phenolato-µ-benzoato-bridged dinuclear copper(II) cluster with a ferromagnetic coupling. X-Ray Struct. Anal. Online 2018, 34, 51–53. [Google Scholar] [CrossRef]
- Kakuta, Y.; Masuda, N.; Kurushima, M.; Hashimoto, T.; Yoshioka, D.; Sakiyama, H.; Hiraoka, Y.; Handa, M.; Mikuriya, M. Synthesis, crystal structures, spectral, electrochemical, and magnetic properties of di-µ-phenoxido-bridged dinuclear copper(II) complexes with N-salicylidene-2-hydroxybenzylamine derivatives: Axial coordination effect of dimethyl sulfoxide molecule. Chem. Pap. 2014, 68, 923–931. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yamakawa, C.; Tanabe, K.; Nukita, R.; Amabe, Y.; Yoshioka, D.; Mitsuhashi, R.; Tatehata, R.; Tanaka, H.; Handa, M. Copper(II) carboxylates with 2,3,4-trimethoxybenzoate and 2,4,6-trimethoxybenzoate: Dinuclear Cu(II) cluster and µ-aqua-bridged Cu(II) chain molecule. Magnetochemistry 2021, 7, 35. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; House, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
- Mautner, F.A.; Koikawa, M.; Mikuriya, M.; Harrelson, E.V.; Massoud, S.S. Copper(II)-azido complexes constructed from polypyridyl amine ligands. Polyhedron 2013, 59, 17–22. [Google Scholar] [CrossRef]
- Mukherjee, J.; Mukherje, R. Catecholase activity of dinuclear copper(II) complexes with variable endogenous and exogenous bridge. Inorg. Chim. Acta 2002, 337, 429–438. [Google Scholar] [CrossRef]
- Karlin, K.D.; Farooq, A.; Hayes, J.C.; Cohen, B.I.; Rowe, T.M.; Sinn, E.; Zubieta, J. Models for met-hemocyanin derivatives: Structural and spectroscopic comparisons of analogous phenolate and X (X = OH−, OMe−, N3−,C1−, OAc−, OBz−) doubly bridged dinuclear copper(II) Complexes. Inorg. Chem. 1987, 26, 1271–1280. [Google Scholar] [CrossRef]
- Karlin, K.D.; Tyeklár, Z. (Eds.) Bioinorganic Chemistry of Copper; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Lubben, M.; Hage, R.; Meetsma, A.; Bÿma, K.; Fering, B.L. Modeling Dinuclear Copper Sites of Biological Relevance: Synthesis, Molecular Structure, Magnetic Properties, and 1H NMR Spectroscopy of a Nonsymmetric Dinuclear Copper(II) Complex. Microcalorimetric Determination of Stepwise Complexation of Copper(II) by a Nonsymmetric Dinucleating Ligand. Inorg. Chem. 1995, 34, 2217–2224. [Google Scholar] [CrossRef]
- Kitajima, N.; Fujisawa, K.; Fujimoto, C.; Morooka, Y.; Hashimoto, S.; Kitagawa, T.; Toriumi, K.; Tatsumi, K.; Nakamura, A. A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of µ-η2.η2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = i-Pr and Ph). J. Am. Chem. Soc. 1992, 114, 1277–1291. [Google Scholar] [CrossRef]
- Fujieda, N.; Umakoshi, K.; Ochi, Y.; Nishikawa, Y.; Yanagisawa, S.; Kubo, M.; Kurisu, G.; Itoh, S. Copper–Oxygen Dynamics in the Tyrosinase Mechanism. Angew. Chem. Int. Ed. 2020, 132, 13487–13492. [Google Scholar] [CrossRef]
- Ma, L.; Lu, L.; Zhu, M.; Wang, Q.; Gao, F.; Yuan, C.; Wu, Y.; Xing, S.; Fu, X.; Mei, Y.; et al. Dinuclear copper complexes of organic claw: Potent inhibition of protein tyrosine phosphatases. J. Inorg. Biochem. 2011, 105, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.; Rossia, L.M.; Horn, A., Jr.; Vencato, I.; Bortoluzzi, A.J.; Zucco, C.; Mangrich, A.S. Synthesis, structure and properties of the first dinuclear copper(II) complex as a structural model for the phenolic intermediate in tyrosinase–cresolase activity. Inorg. Chem. Commun. 1999, 2, 334–337. [Google Scholar] [CrossRef]
- Whittaker, J.W. Metal Ions in Biological Systems; Sigel, H., Sigel, A., Eds.; Marcel Dekker: New York, NY, USA, 1994; Volume 30, pp. 315–360. [Google Scholar]
- Thomas, F.; Gellon, G.; Gautier-Luneau, I.; Saint-Aman, E.; Pierre, J.-L. A Structural and Functional Model of Galactose Oxidase: Control of the One-Electron Oxidized Active Form through Two Differentiated Phenolic Arms in a Tripodal Ligand. Angew. Chem. Int. Ed. 2002, 41, 3047–3050. [Google Scholar] [CrossRef]
- Taki, M.; Kumei, H.; Nagatomo, S.; Kitagawa, T.; Itoh, S.; Fukuzumi, S. Active site models for galactose oxidase containing two different phenol groups. Inorg. Chim. Acta 2000, 300–302, 622–632. [Google Scholar] [CrossRef]
- Sokolowski, A.; Leutbecher, H.; Weyhermüller, T.; Schnepf, R.; Bothe, E.; Bill, E.; Hildebrandt, P.; Wieghardt, K. Phenoxyl-copper(II) complexes: Models for the active site of galactose oxidase. J. Biol. Inorg. Chem. 1997, 2, 444–453. [Google Scholar] [CrossRef]
- Ochs, C.; Hahn, F.E.; Fröhlich, R. Coordination Chemistry of Unsymmetrical Tripodal Ligands with NNO2 Donor Set. Eur. J. Inorg. Chem. 2001, 2427–2436. [Google Scholar] [CrossRef]
- Ito, N.; Phillips, S.E.V.; Yadav, K.D.S.; Knowles, P.F. Crystal Structure of a Free Radical Enzyme, Galactose Oxidase. J. Mol. Biol. 1994, 238, 794–814. [Google Scholar] [CrossRef]
- Mukherjee, D.; Nag, P.; Shteinman, A.A.; Vennapusa, S.R.; Mandal, U.; Mitra, M. Catechol oxidation promoted by bridging phenoxo moieties in a bis(μ-phenoxo)-bridged dicopper(II) complex. RSC Adv. 2021, 11, 22951–22959. [Google Scholar] [CrossRef]
- Patriarca, M.; Daier, V.; Camí, G.; Rivière, E.; Hureau, C.; Signorella, S. Preparation, characterization and activity of CuZn and Cu2 superoxide dismutase mimics encapsulated in mesoporous silica. J. Inorg. Biochem. 2020, 207, 111050. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Das, L.K.; Drew, M.G.B.; Diaz, C.; Ghosh, A. Insertion of a Hydroxido Bridge into a Diphenoxido Dinuclear Copper(II) Complex: Drastic Change of the Magnetic Property from Strong Antiferromagnetic to Ferromagnetic and Enhancement in the Catecholase Activity. Inorg. Chem. 2012, 51, 10111–10121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Zhang, S. Oxidative DNA cleavage promoted by two phenolate-bridged binuclear copper(II) complexes. New J. Chem. 2015, 39, 1814–1821. [Google Scholar] [CrossRef]
- Berthet, N.; Martel-Frachet, V.; Michel, F.; Philouze, C.; Hamman, S.; Ronot, X.; Thomas, F. Nuclease and anti-proliferative activities of copper(II) complexes of N3O tripodal ligands involving a sterically hindered phenolate. Dalton Trans. 2013, 42, 8468–8483. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Salem, N.M.H.; Fischer, R.C.; Torvisco, A.; Mautner, F.A.; Vančo, J.; Belza, J.; Dvořák, Z.; Trávníček, Z. Dinuclear Doubly Bridged Phenoxido Copper(II) Complexes as Efficient Anticancer Agents. Eur. J. Med. Chem. 2023, 246, 114992. [Google Scholar] [CrossRef]
- Jahromi, Z.M.; Asadi, Z.; Eigner, V.; Dusek, M.; Rastegari, B. A new phenoxo-bridged dicopper Schiff base complex: Synthesis, crystal structure DNA/BSA interaction, cytotoxicity assay and catecholase activity. Polyhedron 2022, 221, 115891. [Google Scholar] [CrossRef]
- Ferraresso, L.G.; de Arruda, E.G.R.; de Moraes, T.P.L.; Fazzi, R.B.; Ferreira, A.M.D.C.; Abbehausen, C. Copper(II) and zinc(II) dinuclear enzymes model compounds: The nature of the metal ion in the biological function. J. Mol. Struct. 2017, 1150, 316–328. [Google Scholar] [CrossRef]
- Novoa, N.; Justaud, F.; Hamon, P.; Roisnel, T.; Cador, O.; Guennic, B.L.; Manzur, C.; Carrillo, D.; Hamon, J.-R. Doubly phenoxide-bridged binuclear copper(II) complexes with ono tridentate Schiff base ligand: Synthesis, structural, magnetic and theoretical studies. Polyhedron 2015, 86, 81–88. [Google Scholar] [CrossRef]
- Anbu, S.; Kandaswamy, M. Electrochemical, magnetic, catalytic, DNA binding and cleavage studies of new mono and binuclear copper(II) complexes. Polyhedron 2011, 30, 123–131. [Google Scholar] [CrossRef]
- Assey, G.E.; Yisgedu, T.; Gultneh, Y.; Butcher, R.J.; Tesema, Y. Di-μ-perchlorato-bis-{μ-2-[(2-pyrid-yl)methyl-amino-meth-yl] phenolato)dicopper(II) acetonitrile disolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m1007–m1008. [Google Scholar] [CrossRef]
- Assey, G.E.; Tesema, Y.; Yisgedu, T.; Gultneh, Y.; Butcher, R.J. Bis[μ-2-(2-pyridylmethyl-amino-meth-yl) phenolato]-κN,N′,O:O;κO:N,N′,O-bis-[(thio-cyanato-κN)copper(II)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m1121–m1122. [Google Scholar] [CrossRef]
- Ma, J.-C.; Yang, J.; Ma, J.-F. Bis[μ-2,4-dibromo-6-(2-pyridylmethylaminomethyl) phenolato]bis[nitratocopper(II)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, m2284. [Google Scholar] [CrossRef]
- Choi, K.-Y. Synthesis and Crystal Structure of Phenolato-Bridged Dinuclear Copper(II) Complex with (2-Hydroxybenzyl)(2-pyridylmethyl)amine. J. Chem. Cryst. 2010, 40, 1016–1020. [Google Scholar] [CrossRef]
- Tandon, S.S.; Bunge, S.D.; Patel, N.; Wang, E.C.; Thompson, L.K. Self-Assembly of Antiferromagnetically-Coupled Copper(II) Supramolecular Architectures with Diverse Structural Complexities. Molecules 2020, 25, 5549. [Google Scholar] [CrossRef]
- Adams, H.; Bailey, N.A.; Campbell, I.K.; Fenton, D.E.; He, Q.-Y. Formation of axial phenolate–metal bonds in square-pyramidal complexes. J. Chem. Soc. Dalton Trans. 1996, 2233–2237. [Google Scholar] [CrossRef]
- Mallah, T.; Boillot, M.-L.; Kahn, O.; Gouteron, J.; Jeannin, S.; Jeannin, Y. Crystal Structures and Magnetic Properties of p-Phenolato Copper(I1) Binuclear Complexes with Hydroxo, Azido, and Cyanato-O Exogenous Bridges. Inorg. Chem. 1986, 25, 3058–3065. [Google Scholar] [CrossRef]
- You, X.; Wei, Z. Two multidentate ligands utilizing triazolyl, pyridinyl and phenolate groups as donors for constructing dinuclear copper(II) and iron(III) complexes: Syntheses, structures, and electrochemistry. Inorg. Chim. Acta 2014, 423, 332–339. [Google Scholar] [CrossRef]
- Rajendiran, T.M.; Kannappan, R.; Mahalakshmy, R.; Rajeswari, J.; Venkatesan, R.; Rao, P. New unsymmetrical μ-phenoxo- bridged binuclear copper(II) complexes. Transit. Met. Chem. 2003, 28, 447–454. [Google Scholar] [CrossRef]
- Manzur, J.; Mora, H.; Vega, A.; Spodine, E.; Venegas-Yazigi, D.; Garland, M.T.; El Fallah, M.S.; Escuer, A. Copper(II) complexes with new polypodal ligands presenting axial−equatorial phenoxo bridges {2-[(bis(2-pyridylmethyl)amino)methyl]-4-methylphenol,2-[(bis(2-pyridylmethyl)amino)methyl]-4-methyl-6-(methylthio) phenol}: Examples of ferromagnetically coupled bi- and trinuclear copper(II) complexes. Inorg. Chem. 2007, 46, 6924–6932. [Google Scholar] [CrossRef]
- Kani, Y.; Ohba, S.; Ito, S.; Nishida, Y. Redetermination of bis{[(2-hydroxyphenylmethyl)bis(2-pyridylmethyl)aminato]copper(II)} diperchlorate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, 56, e201. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Torelli, S.; Thomas, F.; Duboc, C.; Philouze, C.; Belle, C.; Hamman, S.; Aman, E.S.; Pierre, J.-L. An Unprecedented Bridging Phenoxyl Radical in Dicopper(II) Complexes: Evidence for an S = 3/2 Spin State. Angew. Chem. Int. Ed. 2005, 44, 438–441. [Google Scholar] [CrossRef]
- Philibert, A.; Thomas, F.; Philouze, C.; Hamman, S.; Saint-Aman, E.; Pierre, J.-L. Galactose Oxidase Models: Tuning the Properties of CuII–Phenoxyl Radicals. Chem. Eur. J. 2003, 9, 3803–3812. [Google Scholar] [CrossRef]
- Wendt, F.; Rolff, M.; Thimm, W.; Näther, C.; Tuczek, F. A Small-molecule Model System of Galactose Oxidase: Geometry, Reactivity, and Electronic Structure. Z. Anorg. Allg. Chem. 2013, 639, 2502–2509. [Google Scholar] [CrossRef]
- Taki, M.; Hattori, H.; Osako, T.; Nagatomo, S.; Shiro, M.; Kitagawa, T.; Itoh, S. Model complexes of the active site of galactose oxidase. Effects of the metal ion binding sites. Inorg. Chim. Acta 2004, 357, 3369–3381. [Google Scholar] [CrossRef]
- Yajima, T.; Shimazaki, Y.; Ishigami, N.; Odani, A.; Yamauchi, O. Conformational preference of the side Conformational preference of the side chain aromatic ring in Cu(II) and Pd(II) complexes of 2N1O-donor ligands. Inorg. Chim. Acta 2002, 337, 193–202. [Google Scholar] [CrossRef]
- Vaidyanathan, M.; Viswanathan, R.; Palaniandavar, M.; Balasubramanian, T.; Prabhaharan, P.; Muthiah, T.P. Copper(II) Complexes with Unusual Axial Phenolate Coordination as Structural Models for the Active Site in Galactose Oxidase: X-ray Crystal Structures and Spectral and Redox Properties of [Cu(bpnp)X] Complexes. Inorg. Chem. 1998, 37, 6418–6427. [Google Scholar] [CrossRef]
- Banerjee, I.; Dolai, M.; Jana, A.D.; Das, K.K.; Ali, M. σ-Aromaticity in dinuclear copper(ii) complexes: Novel interaction between perchlorate anion and σ-aromatic [Cu2X2] (X = N or O) core. CrystEngComm 2012, 14, 4972–4975. [Google Scholar] [CrossRef]
- Thompson, L.K.; Mandal, S.K.; Tandon, S.S.; Bridson, J.N.; Park, M.K. Magnetostructural Correlations in Bis(μ2-phenoxide)-Bridged Macrocyclic Dinuclear Copper(II) Complexes. Influence of Electron-Withdrawing -Withdrawing Substituents on Exchange Coupling. Inorg. Chem. 1996, 35, 3117–3125. [Google Scholar] [CrossRef]
- Yadav, A.; Lama, P.; Bieńko, A.; Bieńko, D.; Siddiqui, K.A. H-bonded supramolecular synthon induced magnetic superexchange phenomenon results weak ferromagnetic and strong antiferromagnetic interactions in two new copper-orotate coordination network. Polyhedron 2018, 141, 247–261. [Google Scholar] [CrossRef]
- Tang, J.; Costa, J.S.; Golobič, A.; Kozlečvar, B.; Robertazzi, A.; Vargiu, A.V.; Gamez, P.; Reedijk, J. Magnetic Coupling between Copper(II) Ions Mediated by Hydrogen-Bonded (Neutral) Water Molecules. Inorg. Chem. 2009, 48, 5473–5479. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Drew, M.G.B.; Asthana, S.; Desplanches, C.; Ghosh, A. Mono-aqua-bridged dinuclear complexes of Cu(II) containing NNO donor Schiff base ligand: Hydrogen-bond-mediated exchange coupling. J. Mol. Struct. 2010, 965, 39–44. [Google Scholar] [CrossRef]
- Talukder, P.; Sen, S.; Mitra, S.; Dahlenberg, L.; Desplanches, C.; Sutter, J.-P. Evidence for Hydrogen-Bond-Mediated Exchange Coupling in an Aqua-Bridged CuII Dimer: Synthesis, Magnetic Study and Correlation with Density Functional Calculations. Eur. J. Inorg. Chem. 2006, 2006, 329–333. [Google Scholar] [CrossRef]
- Okazawa, A.; Ishida, T. Super–superexchange coupling through a hydrogen bond in a linear copper(II) complex, [Cu(LH)(L)]·BF4·2H2O (LH = N-tert-butyl-N-2-pyridylhydroxylamine). Chem. Phys. Lett. 2009, 480, 198–202. [Google Scholar] [CrossRef]
- Valigura, D.; Moncol, J.; Korabik, M.; Pucekova, Z.; Lis, T.; Mrozinski, J.; Melnik, M. New Dimeric Copper(II) Complex [Cu(5-MeOsal)2(μ-nia)(H2O)]2 with Magnetic Exchange Interactions through H-Bonds. Eur. J. Inorg. Chem. 2006, 3813–3817. [Google Scholar] [CrossRef]
- Desplanches, C.; Ruiz, E.; Rodriguez-Fortea, A.; Alvarez, S. Exchange Coupling of Transition-Metal Ions through Hydrogen Bonding: A Theoretical Investigation. J. Am. Chem. Soc. 2002, 124, 5197–5205. [Google Scholar] [CrossRef]
- Plass, W.; Pohlmann, A.; Rautengarten, J. Magnetic Interactions as Supramolecular Function: Structure and Magnetic Properties of Hydrogen-Bridged Dinuclear Copper(II) Complexes. Angew. Chem. Int. Ed. 2001, 40, 4207–4210. [Google Scholar] [CrossRef]
- Moreno, J.M.; Ruiz, J.; Dominguez-Vera, J.M.; Colacio, E. Spectroscopic and magnetic properties, and crystal structure of a dimer copper(II) complex via hydrogen bonding with a weak ferromagnetic interaction. Inorg. Chim. Acta 1993, 208, 111–115. [Google Scholar] [CrossRef]
- Colacio, E.; Costes, J.P.; Kivekas, R.; Laurent, J.P.; Ruiz, J.; Sundberg, M. A new hydrogen-bonded dinuclear complex involving copper(II) ions in a pseudotetrahedral N3O environment: Molecular and crystal structure and magnetic and spectroscopic properties. Inorg. Chem. 1991, 30, 1475–1479. [Google Scholar] [CrossRef]
- Oberhausen, K.J.; Richardson, J.F.; Buchanan, R.M.; McCusker, J.K.; Hendrickson, D.N.; Latour, J.M. Synthesis and characterization of dinuclear copper(II) complexes of the dinucleating ligand 2,6-bis[(bis((1-methylimidazol-2-yl)methyl)amino)methyl]-4-methylphenol. Inorg. Chem. 1991, 30, 1357–1365. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Rashmawi, L.G.; Louka, F.R.; Massoud, S.S. Structural characterization of metal(II) thiocyanato complexes derived from bis(2-(H-pyrazol-1-yl)ethyl)amine. Polyhedron 2017, 124, 237–242. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, R.F.; David, R.N.; Dartez, M.J.; Nguyn, Q.L.; Labry, N.J.; Fischer, R.C.; Mautner, F.A. Five-coordinate metal(II) complexes based pyrazolyl ligands. Polyhedron 2015, 90, 258–265. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Rijin, J.V.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Archana, V.; Imamura, Y.; Sakiyama, H.; Hada, M. Correlating Magnetic Exchange in Dinuclear Bis(phenolate)-Bridged Com-plexes: A Computational Perspective. Bull. Chem. Soc. Jpn. (BCSJ) 2016, 89, 657–665. [Google Scholar] [CrossRef]
- Venegas-Yazigi, D.; Aravena, D.; Spodine, E.; Ruiz, E.; Alvarez, S. Structural and electronic effects on the exchange interactions in dinuclear bis(phenoxo)-bridged copper(II) complexes. Coord. Chem. Rev. 2010, 254, 2086–2095. [Google Scholar] [CrossRef]
- Mondal, D.; Majee, M.C.; Bhattacharya, K.; Long, J.; Larionova, J.; Khusniyarov, M.M.; Chaudhury, M. Crossover from Antiferromagnetic to Ferromagnetic Exchange Coupling in a New Family of Bis-(μ-phenoxido)dicopper(II) Complexes: A Comprehensive Magneto-Structural Correlation by Experimental and Theoretical Study. ACS Omega. 2019, 4, 10558–10570. [Google Scholar] [CrossRef]
- Mondal, D.; Majee, M.C.; Bhattacharya, K.; Long, J.; Larionova, J.; Khusniyarov, M.M.; Chaudhury, M. Synthesis and characterization of binuclear [ONOX]-type amine-bis(phenolate) copper(II) complexes. Inorg. Chim. Acta 2011, 375, 158–165. [Google Scholar]
- Chaudhuri, P.; Wagner, R.; Weyhermüller, T. Ferromagnetic vs. antiferromagnetic coupling in bis(μ-phenoxo)dicopper(II) complexes. Tuning of the nature of exchange coupling by remote ligand substituents. Inorg. Chem. 2007, 46, 5134–5136. [Google Scholar] [CrossRef] [PubMed]
- Van Crawford, H.; Richardson, H.W.; Wasson, J.R.; Hodgson, D.J.; Hatfield, W.E. Relation between the singlet-triplet splitting and the copper-oxygen-copper bridge angle in hydroxo-bridged copper dimers. Inorg. Chem. 1976, 15, 2107–2110. [Google Scholar] [CrossRef]
- Merz, L.; Hasse, W. Exchange interaction in tetrameric oxygen-bridged copper clusters of the cubane type. Dalton Trans. 1980, 875–879. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: Cambridge, UK, 1993; Chapter 1. [Google Scholar]
- Bruker. SAINT, version 7.23; Bruker: Billerica, MA, USA, 2005. [Google Scholar]
- Bruker. APEX 2, version 2.0-2; Bruker: Billerica, MA, USA, 2006. [Google Scholar]
- Sheldrick, G.M. SADABS, version 2; University of Goettingen: Goettingen, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, T.; van de Streek, J.J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Speck, A.L. PLATON, a Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2001. [Google Scholar]










| 3 | |||
|---|---|---|---|
| Cu1-N1 | 2.089(10) | Cu2-N3 | 2.160(10) |
| Cu1-N2 | 2.325(10) | Cu2-N4 | 2.180(10) |
| Cu1-O1 | 1.892(8) | Cu2-O3 | 1.905(8) |
| Cu1-O2 | 1.963(9) | Cu2-O2 | 2.002(8) |
| Cu1-O4 | 2.013(8) | Cu2-O4 | 1.999(8) |
| Cu1-O2-Cu2 | 100.4(4) | O2-Cu1-O4 | 75.4(3) |
| Cu1-O4-Cu2 | 98.8(4) | O2-Cu2-O4 | 74.8(4) |
| O1-Cu1-O2 | 157.0(4) | O3-Cu2-O4 | 164.3(4) |
| O4-Cu1-N1 | 160.4(4) | O2-Cu2-N4 | 142.0(4) |
| 4 | |||
| Cu1-N1 | 2.0627(19) | Cu2-N3 | 2.090(2) |
| Cu1-N2 | 2.449(2) | Cu2-N4 | 2.3532(19) |
| Cu1-O1 | 1.8783(17) | Cu2-O3 | 1.8805(17) |
| Cu1-O2 | 1.9580(17) | Cu2-O2 | 2.0349(16) |
| Cu1-O4 | 2.0218(16) | Cu2-O4 | 1.9465(16) |
| Cu1-O2-Cu2 | 97.17(7) | O2-Cu1-O4 | 75.78(7) |
| Cu1-O4-Cu2 | 97.98(7) | O2-Cu2-O4 | 75.73(7) |
| O1-Cu1-O2 | 160.59(7) | O3-Cu2-O4 | 165.55(7) |
| O4-Cu1-N1 | 156.88(7) | O2-Cu2-N4 | 138.62(7) |
| 9 | |||
| Cu1-N1 | 2.054(2) | Cu3-N3 | 2.066(2) |
| Cu1-N2 | 2.351(2) | Cu3-N4 | 2.324(2) |
| Cu1-O1 | 1.929(2) | Cu3-O4 | 1.941(2) |
| Cu1-O2 | 2.000(2) | Cu3-O5 | 1.985(2) |
| Cu1-O3 | 1.957(2) | Cu3-O6 | 1.944(2) |
| Cu2-O2 | 1.9488(19) | Cu2-O3 | 1.999(2) |
| Cu2-O4 | 1.920(2) | Cu2-O5 | 1.959(2) |
| Cu1-O2-Cu2 | 97.33(9) | O2-Cu1-O3 | 75.43(8) |
| Cu1-O3-Cu2 | 100.19(9) | O2-Cu2-O3 | 77.73(8) |
| Cu2-O4-Cu3 | 99.27(9) | O4-Cu2-O5 | 77.91(8) |
| Cu2-O5-Cu3 | 96.48(9) | O4-Cu3-O5 | 76.79(8) |
| O2-Cu2-O4 | 102.80(8) | O3-Cu2-O5 | 103.85(9) |
| O1-Cu1-O2 | 161.11(8) | O5-Cu3-O6 | 153.32(9) |
| O3-Cu1-N1 | 161.70(8) | O4-Cu3-N3 | 167.39(9) |
| Complex | J (cm−1) | C.N. (Geom., τ5 or τ4) | Cu…Cu (Å) | Cu-O-Cu’ (°) | Cu-O-Cu-O (°) | Cu-O-C-C (°) | τ (°) |
|---|---|---|---|---|---|---|---|
| 1 | −289 | 5 (SP, 0.23) | 3.092 | 103.44 | 15.9 | 52.1 | 7.8 |
| 2 | −202 | 5 (SP, 0.24) | 3.006 | 99.12 | 24.9 | 57.3 | 3.6 |
| 3 | −145 | 5 (SP, 0.06/0.37) | 3.047 | 98.8, 100.4 | 24.8 | 50.9, 71.0 | 10.5, 12.2 |
| 4 | −146 | 5(SP, 0.07/0.45) | 2.995 | 97.17, 97.78 | 26.9 | 52.5, 67.5 | 3.2, 12.8 |
| 5 | −176 | 4(SQP, 0.17) 5(SP, 0.011) | 2.9331 | 96.70, 97.58 | 29.4 | 50.5, 49.9 | 2.7, 5.5 |
| 6 | −250 | 4(SQP, 0.17) 5(SP, 0.013) | 2.941 | 97.56, 96.93 | 29.8 | 49.9, 51.7 | 6.5, 4.6 |
| 7 | −3.6 | 5(SP, 0.41) + 1 | 3.130 | 97.72 | 0 | 11.3 | 31.3 |
| 8 | −4.6 | 5(SP, 0.40) + 1 | 3.098 | 96.63 | 0 | 11.3 | 30.9 |
| 9 | −59 | 4(SQP, 0.17) 5(SP: 0.01, 0.23) | 2.9652 2.9418 | 96.48, 97.33, 99.27, 100.19 | 22.9, 23.5 | 44.2, 41.9 | 8.2, 20.0 |
| 10 | +3.5 | 4(SQP, 0.23) | 7.269 |
| Compound | 3 | 4 | 9 |
|---|---|---|---|
| Empirical formula | C40H48Br4Cu2N4O4 | C51H74Cu2N4O5 | C47H62Cl4Cu3N4O7 |
| Formula mass | 1095.53 | 950.22 | 1131.45 |
| System | Monoclinic | Triclinic | Monoclinic |
| Space group | P21/c | P-1 | P21/n |
| a (Å) | 15.433(3) | 12.6538(12) | 10.7647(5) |
| b (Å) | 14.198(3) | 13.0192(12) | 29.4374(14) |
| c (Å) | 20.973(4) | 15.4646(15) | 16.5922(8) |
| α (°) | 90 | 95.347(5) | 90 |
| β (°) | 100.460(7) | 101.633(5) | 90.673(2) |
| γ (°) | 90 | 103.880(4) | 90 |
| V (Å3) | 4519.2(16) | 2395.5(4) | 5275.5(4) |
| Z | 4 | 2 | 4 |
| θ max (°) | 26.000 | 30.558 | 24.999 |
| Data collected | 82442 | 133738 | 159804 |
| Unique refl. | 8840 | 14144 | 9251 |
| Parameters | 495 | 573 | 600 |
| Goodness-of-fit on F2 | 1.069 | 1.057 | 1.109 |
| R1/wR2 (all data) | 0.1102/0.2845 | 0.0528/0.1336 | 0.0357/0.0923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massoud, S.S.; Louka, F.R.; Dial, M.T.; Salem, N.N.M.H.; Fischer, R.C.; Torvisco, A.; Mautner, F.A.; Nakashima, K.; Handa, M.; Mikuriya, M. Magnetostructural Properties of Some Doubly-Bridged Phenoxido Copper(II) Complexes. Molecules 2023, 28, 2648. https://doi.org/10.3390/molecules28062648
Massoud SS, Louka FR, Dial MT, Salem NNMH, Fischer RC, Torvisco A, Mautner FA, Nakashima K, Handa M, Mikuriya M. Magnetostructural Properties of Some Doubly-Bridged Phenoxido Copper(II) Complexes. Molecules. 2023; 28(6):2648. https://doi.org/10.3390/molecules28062648
Chicago/Turabian StyleMassoud, Salah S., Febee R. Louka, Madison T. Dial, Nahed N. M. H. Salem, Roland C. Fischer, Ana Torvisco, Franz A. Mautner, Kai Nakashima, Makoto Handa, and Masahiro Mikuriya. 2023. "Magnetostructural Properties of Some Doubly-Bridged Phenoxido Copper(II) Complexes" Molecules 28, no. 6: 2648. https://doi.org/10.3390/molecules28062648
APA StyleMassoud, S. S., Louka, F. R., Dial, M. T., Salem, N. N. M. H., Fischer, R. C., Torvisco, A., Mautner, F. A., Nakashima, K., Handa, M., & Mikuriya, M. (2023). Magnetostructural Properties of Some Doubly-Bridged Phenoxido Copper(II) Complexes. Molecules, 28(6), 2648. https://doi.org/10.3390/molecules28062648