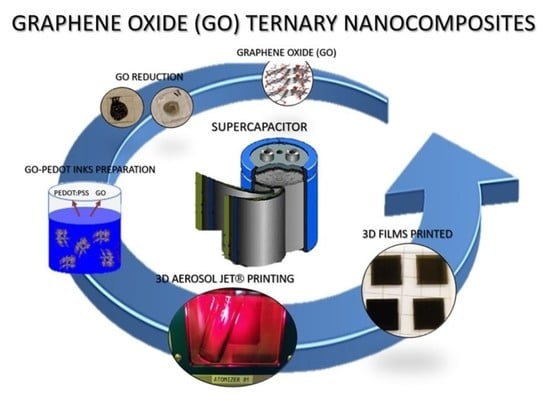
Pedot:PSS/Graphene Oxide (GO) Ternary Nanocomposites for Electrochemical Applications
Abstract
:1. Introduction
State of Purpose
2. Results and Discussion
2.1. Graphene Oxide Characterisation
2.2. Graphene Oxide Reduction by Green Agents
2.3. Inks Characterisation
2.4. Ternary Nanocomposite Electrochemical Characterization
2.5. Aerosol Jet® Printing of Ternary Nanocomposite
3. Materials and Methods
3.1. Materials
3.2. Ink Preparation
3.3. Inks Characterisation Techniques
3.4. Graphene Oxide Characterisation and Reduction by Green Additives
3.5. Nanocomposites Characterisation
3.6. Films Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Willner, I. Biomaterials for Sensors, Fuel Cells, and Circuitry. Science 2002, 298, 2407–2408. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [Green Version]
- Uz, M.; Mallapragada, S.K. Conductive Polymers and Hydrogels for Neural Tissue Engineering. J. Indian Inst. Sci. 2019, 99, 489–510. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e17–e35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, R. Advances in conductive polymers. Eur. Polym. J. 1998, 34, 1053–1060. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Islam, M.; Chidembo, A.T.; Aboutalebi, S.H.; Cardillo, D.; Liu, H.K.; Konstantinov, K.; Dou, S.X. Liquid Crystalline Graphene Oxide/PEDOT:PSS Self-Assembled 3D Architecture for Binder-Free Supercapacitor Electrodes. Front. Energy Res. 2014, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- FEOLA, A. Caratterizzazione Delle Proprietà Elettriche Dell’ossido di Grafene Ridotto Mediante Acido Ascorbico. 2018. Available online: http://hdl.handle.net/10589/139164 (accessed on 8 January 2023).
- Wang, X.; Zhi, L.; Müllen, K. Transparent, Conductive Graphene Electrodes for Dye-Sensitized Solar Cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Muda, M.R.; Ramli, M.M.; Isa, S.S.M.; Jamlos, M.F.; Murad, S.A.Z.; Norhanisah, Z.; Isa, M.M.; Kasjoo, S.R.; Ahmad, N.; Nor, N.I.M. Fundamental study of reduction graphene oxide by sodium borohydride for gas sensor application. In AIP Conference Proceedings; AIP Publishing LLC: Hoboken, NJ, USA, 2017; p. 020034. [Google Scholar]
- Ding, Y.H.; Zhang, P.; Zhuo, Q.; Ren, H.M.; Yang, Z.M.; Jiang, Y. A green approach to the synthesis of reduced graphene oxide nanosheets under UV irradiation. Nanotechnology 2011, 22, 215601. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Hua, Y.; Sun, M.; Ma, N. The mechanism of the reaction of graphite oxide to reduced graphene oxide under ultraviolet irradiation. Carbon 2013, 54, 412–418. [Google Scholar] [CrossRef]
- Giuri, A.; Masi, S.; Colella, S.; Listorti, A.; Rizzo, A.; Gigli, G.; Liscio, A.; Treossi, E.; Palermo, V.; Rella, S.; et al. UV Reduced Graphene Oxide PEDOT:PSS Nanocomposite for Perovskite Solar Cells. IEEE Trans. Nanotechnol. 2016, 15, 725–730. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Bhargava, R.; Khan, S.; Ansari, M.M.N.; Ahmad, N. Green synthesis approach for the reduction of graphene oxide by using glucose. In AIP Conference Proceedings; AIP Publishing LLC: Hoboken, NJ, USA, 2019; p. 030075. [Google Scholar] [CrossRef]
- Giuri, A.; Colella, S.; Listorti, A.; Rizzo, A.; Mele, C.; Corcione, C.E. GO/glucose/PEDOT:PSS ternary nanocomposites for flexible supercapacitors. Compos. Part B Eng. 2018, 148, 149–155. [Google Scholar] [CrossRef]
- Giuri, A.; Masi, S.; Colella, S.; Kovtun, A.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Esposito Corcione, C.; Rizzo, A.; Listorti, A. Cooperative effect of GO and glucose on PEDOT: PSS for high VOC and hysteresis-free solution-processed perovskite solar cells. Adv. Funct. Mater. 2016, 26, 6985–6994. [Google Scholar] [CrossRef]
- Dua, V.; Surwade, S.P.; Ammu, S.; Agnihotra, S.R.; Jain, S.; Roberts, K.E.; Park, S.; Ruoff, R.S.; Manohar, S.K. All-Organic Vapor Sensor Using Inkjet-Printed Reduced Graphene Oxide. Angew. Chem. Int. Ed. 2010, 49, 2154–2157. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, S.; Chen, J.-T.; Hu, X.-P.; Du, Z.-F.; Qiu, Y.-X.; Zhao, D.-L. Reduction of graphene oxide at room temperature with vitamin C for RGO–TiO2 photoanodes in dye-sensitized solar cell. Thin Solid Films 2015, 584, 29–36. [Google Scholar] [CrossRef]
- Khasim, S.; Pasha, A.; Badi, N.; Lakshmi, M.; Mishra, Y.K. High performance flexible supercapacitors based on secondary doped PEDOT–PSS–graphene nanocomposite films for large area solid state devices. RSC Adv. 2020, 10, 10526–10539. [Google Scholar] [CrossRef]
- Abd Alreda, B.; Al-Rubaye, S.H. Study of Electrochemical Properties of NiCo2O4/Reduced Graphene Oxide/PEDOT: PSS Ternary Nanocomposite for High Performance Supercapacitor Electrode. NeuroQuantology 2021, 19, 77–83. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, J.; Yang, D.; Sun, P.; Li, X. Excellent performance of flexible supercapacitor based on the ternary composites of reduced graphene oxide/molybdenum disulfide/poly (3,4-ethylenedioxythiophene). Electrochim. Acta 2020, 330, 135205. [Google Scholar] [CrossRef]
- Giuri, A.; Striani, R.; Carallo, S.; Colella, S.; Rizzo, A.; Mele, C.; Bagheri, S.; Seiti, M.; Ferraris, E.; Corcione, C.E. Waste carbon ashes/PEDOT:PSS nano-inks for printing of supercapacitors. Electrochim. Acta 2023, 441, 141780. [Google Scholar] [CrossRef]
- Wilkinson, N.J.; Smith, M.A.A.; Kay, R.W.; Harris, R.A. A review of aerosol jet printing—A non-traditional hybrid process for micro-manufacturing. Int. J. Adv. Manuf. Technol. 2019, 105, 4599–4619. [Google Scholar] [CrossRef] [Green Version]
- Gibney, R.; Patterson, J.; Ferraris, E. High-Resolution Bioprinting of Recombinant Human Collagen Type III. Polymers 2021, 13, 2973. [Google Scholar] [CrossRef]
- Seiti, M.; Ginestra, P.S.; Ferraro, R.M.; Giliani, S.; Vetrano, R.M.; Ceretti, E.; Ferraris, E. Aerosol Jet® Printing of Poly (3, 4-Ethylenedioxythiophene): Poly (Styrenesulfonate) onto Micropatterned Substrates for Neural Cells In Vitro Stimulation. Int. J. Bioprint. 2022, 8, 504. [Google Scholar] [CrossRef]
- Seiti, M.; Degryse, O.; Ferraris, E. Aerosol Jet® printing 3D capabilities for metal and polymeric inks. Mater. Today Proc. 2022, 70, 38–44. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, X.; Guo, Z.; Cai, C.; Chen, Z.; Wexler, D.; Li, S.; Liu, H.K. Carbon-coated SnO2/graphene nanosheets as highly reversible anode materials for lithium ion batteries. Carbon 2012, 50, 1897–1903. [Google Scholar] [CrossRef]
- Acocella, M.R.; Mauro, M.; Falivene, L.; Cavallo, L.; Guerra, G. Inverting the Diastereoselectivity of the Mukaiyama–Michael Addition with Graphite-Based Catalysts. ACS Catal. 2014, 4, 492–496. [Google Scholar] [CrossRef]
- Wong, C.P.P.; Lai, C.W.; Lee, K.M.; Hamid, S.B.A. Advanced Chemical Reduction of Reduced Graphene Oxide and Its Photocatalytic Activity in Degrading Reactive Black 5. Materials 2015, 8, 7118–7128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide vial-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Pantwalawalkar, J.; More, H.; Bhange, D.; Patil, U.; Jadhav, N. Novel curcumin ascorbic acid cocrystal for improved solubility. J. Drug Deliv. Sci. Technol. 2021, 61, 102233. [Google Scholar] [CrossRef]
- Gu, Z.-G.; Li, D.-J.; Zheng, C.; Kang, Y.; Wöll, C.; Zhang, J. MOF-Templated Synthesis of Ultrasmall Photoluminescent Carbon-Nanodot Arrays for Optical Applications. Angew. Chem. Int. Ed. 2017, 56, 6853–6858. [Google Scholar] [CrossRef] [PubMed]
- Ashok, R.P.R.; Thomas, M.S.; Varughese, S. Multi-region to single region shear thinning transitions in drying PEDOT:PSS dispersions: Contributions from charge density fluctuations. Soft Matter 2015, 11, 8441–8451. [Google Scholar] [CrossRef]
- Aho, J.; Syrjälä, S. On the measurement and modeling of viscosity of polymers at low temperatures. Polym. Test. 2008, 27, 35–40. [Google Scholar] [CrossRef]
- Cross, M.M. Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]
- Lall, P.; Goyal, K.; Kothari, N.; Leever, B.; Miller, S. Effect of Process Parameters on Aerosol Jet Printing of Multi-Layer Circuitry. In International Electronic Packaging Technical Conference and Exhibition; American Society of Mechanical Engineers: Auburn, AL, USA, 2019; p. V001T04A005. [Google Scholar] [CrossRef]
- Salary, R.; Lombardi, J.P.; Tootooni, M.S.; Donovan, R.; Rao, P.K.; Poliks, M.D. In Situ Sensor-Based Monitoring and Computational Fluid Dynamics (CFD) Modeling of Aerosol Jet Printing (AJP) Process. In International Manufacturing Science and Engineering Conference; American Society of Mechanical Engineers: Auburn, AL, USA, 2016; p. V002T04A049. [Google Scholar] [CrossRef]
- Arsenov, P.; Efimov, A.; Ivanov, V. Optimizing Aerosol Jet Printing Process of Platinum Ink for High-Resolution Conductive Microstructures on Ceramic and Polymer Substrates. Polymers 2021, 13, 918. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Say, M.G.; Brooke, R.; Beni, V.; Nilsson, D.; Lassnig, R.; Berggren, M.; Edberg, J.; Engquist, I. Upscalable ultra thick rayon carbon felt based hybrid organic-inorganic electrodes for high energy density supercapacitors. Energy Storage 2022, 4, e348. [Google Scholar] [CrossRef]
- Mele, C.; Catalano, M.; Taurino, A.; Bozzini, B. Electrochemical fabrication of nanoporous gold-supported manganese oxide nanowires based on electrodeposition from eutectic urea/choline chloride ionic liquid. Electrochim. Acta 2013, 87, 918–924. [Google Scholar] [CrossRef]
- Gao, Y. Graphene and Polymer Composites for Supercapacitor Applications: A Review. Nanoscale Res. Lett. 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Wilamowska, M.; Kujawa, M.; Michalska, M.; Lipińska, L.; Lisowska-Oleksiak, A. Electroactive polymer/graphene oxide nanostructured composites; evidence for direct chemical interactions between PEDOT and GOx. Synth. Met. 2016, 220, 334–346. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Bozzini, B.; Mele, C.; D’Urzo, L. Electrodeposition of Cu from Acidic Sulphate Solutions in the Presence of PEG—Part II Visible Electroreflectance Spectroscopy Measurements during Electrodeposition. J. Appl. Electrochem. 2006, 36, 87–96. [Google Scholar] [CrossRef]
- Chang, J.-K.; Huang, C.-H.; Tsai, W.-T.; Deng, M.-J.; Sun, I.-W.; Chen, P.-Y. Manganese films electrodeposited at different potentials and temperatures in ionic liquid and their application as electrode materials for supercapacitors. Electrochim. Acta 2008, 53, 4447–4453. [Google Scholar] [CrossRef]
- Chang, J.-K.; Huang, C.-H.; Lee, M.-T.; Tsai, W.-T.; Deng, M.-J.; Sun, I.-W. Physicochemical factors that affect the pseudocapacitance and cyclic stability of Mn oxide electrodes. Electrochim. Acta 2009, 54, 3278–3284. [Google Scholar] [CrossRef]
- Bozzini, B.; Gianoncelli, A.; Kaulich, B.; Mele, C.; Prasciolu, M.; Kiskinova, M. Electrodeposition of manganese oxide from eutectic urea/choline chloride ionic liquid: An in situ study based on soft X-ray spectromicroscopy and visible reflectivity. J. Power Sources 2012, 211, 71–76. [Google Scholar] [CrossRef]
- Ryu, K.S.; Lee, Y.-G.; Hong, Y.-S.; Park, Y.J.; Wu, X.; Kim, K.M.; Kang, M.G.; Park, N.-G.; Chang, S.H. Poly(ethylenedioxythiophene) (PEDOT) as polymer electrode in redox supercapacitor. Electrochim. Acta 2004, 50, 843–847. [Google Scholar] [CrossRef]
- Sharma, R.K.; Zhai, L. Multiwall carbon nanotube supported poly(3,4-ethylenedioxythiophene)/manganese oxide nano-composite electrode for super-capacitors. Electrochim. Acta 2009, 54, 7148–7155. [Google Scholar] [CrossRef]
- Han, Y.; Ding, B.; Tong, H.; Zhang, X. Capacitance properties of graphite oxide/poly(3,4-ethylene dioxythiophene) composites. J. Appl. Polym. Sci. 2011, 121, 892–898. [Google Scholar] [CrossRef]
- Huang, H.; Xia, L.; Zhao, Y.; Zhang, H.; Cong, T.; Wang, J.; Wen, N.; Yang, S.; Fan, Z.; Pan, L. Three-dimensional porous reduced graphene oxide/PEDOT:PSS aerogel: Facile preparation and high performance for supercapacitor electrodes. Electrochim. Acta 2020, 364, 137297. [Google Scholar] [CrossRef]
- Hong, K.; Kim, S.H.; Mahajan, A.; Frisbie, C.D. Aerosol Jet Printed p- and n-type Electrolyte-Gated Transistors with a Variety of Electrode Materials: Exploring Practical Routes to Printed Electronics. ACS Appl. Mater. Interfaces 2014, 6, 18704–18711. [Google Scholar] [CrossRef] [PubMed]
- Tarabella, G.; Vurro, D.; Lai, S.; D’Angelo, P.; Ascari, L.; Iannotta, S. Aerosol jet printing of PEDOT:PSS for large area flexible electronics. Flex. Print. Electron. 2020, 5, 014005. [Google Scholar] [CrossRef]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S.; Imran, M. A green method for the synthesis of Copper Nanoparticles using L-ascorbic acid. Matéria (Rio J.) 2014, 19, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Data Sheet. Available online: https://www.sigmaaldrich.com/IT/en/sds/aldrich/483095 (accessed on 9 January 2023).
- Data Sheet. Available online: https://www.sigmaaldrich.com/IT/en/sds/aldrich/763705 (accessed on 9 January 2023).
- Data Sheet. Available online: https://www.sigmaaldrich.com/IT/en/sds/aldrich/158968 (accessed on 9 January 2023).
- Data Sheet. Available online: https://www.sigmaaldrich.com/IT/en/sds/sigma/a4544 (accessed on 9 January 2023).








| Sample | η0 (Pa∙s) | η∞ (Pa∙s) | τ | m |
|---|---|---|---|---|
| PEDOT | 1.1 | 0.007 | 4.78 | 1.24 |
| GO-PEDOT | 1.5 | 0.006 | 2.92 | 1.10 |
| GGO-PEDOT | 2.1 | 0.008 | 4.82 | 0.97 |
| AAGO-PEDOT | 1.6 | 0.006 | 3.19 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, G.; Giuri, A.; Bagheri, S.; Seiti, M.; Degryse, O.; Rizzo, A.; Mele, C.; Ferraris, E.; Corcione, C.E. Pedot:PSS/Graphene Oxide (GO) Ternary Nanocomposites for Electrochemical Applications. Molecules 2023, 28, 2963. https://doi.org/10.3390/molecules28072963
Greco G, Giuri A, Bagheri S, Seiti M, Degryse O, Rizzo A, Mele C, Ferraris E, Corcione CE. Pedot:PSS/Graphene Oxide (GO) Ternary Nanocomposites for Electrochemical Applications. Molecules. 2023; 28(7):2963. https://doi.org/10.3390/molecules28072963
Chicago/Turabian StyleGreco, Giuseppe, Antonella Giuri, Sonia Bagheri, Miriam Seiti, Olivier Degryse, Aurora Rizzo, Claudio Mele, Eleonora Ferraris, and Carola Esposito Corcione. 2023. "Pedot:PSS/Graphene Oxide (GO) Ternary Nanocomposites for Electrochemical Applications" Molecules 28, no. 7: 2963. https://doi.org/10.3390/molecules28072963
APA StyleGreco, G., Giuri, A., Bagheri, S., Seiti, M., Degryse, O., Rizzo, A., Mele, C., Ferraris, E., & Corcione, C. E. (2023). Pedot:PSS/Graphene Oxide (GO) Ternary Nanocomposites for Electrochemical Applications. Molecules, 28(7), 2963. https://doi.org/10.3390/molecules28072963