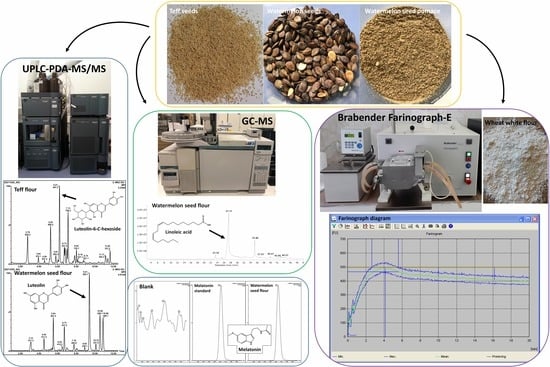
Mineral, Nutritional, and Phytochemical Composition and Baking Properties of Teff and Watermelon Seed Flours
Abstract
:1. Introduction
- -
- determining the nutritional and health benefits of using teff and watermelon seed flour (including whole seed and pomace flour) to enrich white flour by analyzing the mineral, nutritional, phytochemical, and antioxidant composition;
- -
- determining the suitability of teff and watermelon seed flours in combination with refined flour for baking purposes by analyzing the rheological parameters of the dough based on mixtures of flours.
2. Results and Discussion
2.1. Mineral Content
2.2. Protein and Lipid Content
2.3. Phytochemical Screening
2.3.1. UHPLC-PDA-MS/MS Analysis
2.3.2. Total Phenolic (TPC) and Flavonoid Content
2.3.3. GC-MS Analysis
2.3.4. Melatonin (MEL) Content
2.4. Antioxidant Properties of Teff and Watermelon Seed Material
2.5. Farinographic Evaluation of Dough Based on Wheat Flour with Test Supplements
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Mineral Content
3.4. Protein and Lipid Content
3.5. Phytochemical Analyses
3.5.1. Preparation of Extracts
3.5.2. Phytochemical Profiling and Quantification of Flavonoids Using UHPLC-PDA-MS/MS Analysis
3.5.3. Total Phenolic Content (TPC)
3.5.4. GC-MS Analysis
3.5.5. Quantitative UHPLC-MS/MS Determination of Melatonin (MEL)
3.6. Antioxidant Properties of Teff and Watermelon Material
3.6.1. ABTS Assay
3.6.2. DPPH Assay
3.6.3. Ferrous Ion Chelating Activity (FCA)
3.7. Farinographic Evaluation of Dough Based on Wheat Flour with Test Supplements
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ertl, K.; Goessler, W. Grains, whole flour, white flour, and some final goods: An elemental comparison. Eur. Food Res. Technol. 2018, 244, 2065–2075. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jian, C. Sustainable plant-based ingredients as wheat flour substitutes in bread making. Npj Sci. Food 2022, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Jacobs, D.; Marquart, L. Grain Processing and Nutrition. Crit. Rev. Biotechnol. 2001, 21, 49–66. [Google Scholar] [CrossRef]
- Oghbaei, M.; Prakash, J. Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent Food Agric. 2016, 2, 1136015. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.S.; Wang, P.; Wang, N.; Yang, S.; Xiao, Z. Technologies for enhancement of bioactive components and potential health benefits of cereal and cereal-based foods: Research advances and application challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chatzidimitriou, E.; Wood, L.; Hasanalieva, G.; Markellou, E.; Iversen, P.O.; Seal, C.; Baranski, M.; Vigar, V.; Ernst, L.; et al. Effect of wheat species (Triticum aestivum vs. T. spelta), farming system (organic vs conventional) and flour type (wholegrain vs white) on composition of wheat flour—Results of a retail survey in the UK and Germany—2. Antioxidant activity, and phenolic and mineral content. Food Chem. 2020, 6, 100091. [Google Scholar] [CrossRef]
- Zuchowski, J.; Jonczyk, K.; Pecio, L.; Oleszek, W. Phenolic acid concentrations in organically and conventionally cultivated spring and winter wheat. J. Sci. Food Agric. 2011, 91, 1089–1095. [Google Scholar] [CrossRef]
- Mancebo, C.M.; Picón, J.; Gómez, M. Effect of flour properties on the quality characteristics of gluten free sugar-snap cookies. LWT Food Sci. Technol. 2015, 64, 264–269. [Google Scholar] [CrossRef]
- Conte, P.; Fadda, C.; Drabińska, N.; Krupa-Kozak, U. Technological and Nutritional Challenges, and Novelty in Gluten-Free Breadmaking—A Review. Pol. J. Food Nutr. Sci. 2019, 69, 5–21. [Google Scholar] [CrossRef]
- Dziki, D.; Różyło, R.; Gawlik-Dziki, U.; Świeca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Garutti, M.; Nevola, G.; Mazzeo, R.; Cucciniello, L.; Totaro, F.; Bertuzzi, C.A.; Caccialanza, R.; Pedrazzoli, P.; Puglisi, F. The Impact of Cereal Grain Composition on the Health and Disease Outcomes. Front. Nutr. 2022, 9, 888974. [Google Scholar] [CrossRef]
- Zięć, G.; Gambuś, H.; Lukasiewicz, M.; Gambuś, F. Wheat Bread Fortification: The Supplement of Teff Flour and Chia Seeds. Appl. Sci. 2021, 11, 5238. [Google Scholar] [CrossRef]
- Saeid, A.; Ahmed, M. A Review on Effects of Pseudo Cereals Flour on Quality Properties of Biscuit, Cookies and Cake. In Innovation in the Food Sector Through the Valorization of Food and Agro-Food By-Products; Novo de Barros, A., Gouvinhas, I., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Katyal, M.; Kaur, A.; Singh, N. Effects of incorporation of groundnut oil and hydrogenated fat on pasting and dough rheological properties of flours from wheat varieties. J. Food Sci. Technol. 2019, 56, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Mihiretu, A.; Asresu, M. Inclusive technology performance evaluation in the production of teff (Eragrostis tef (Zucc.) Trotter). Adv. Agric. 2022, 2022, 9031999. [Google Scholar] [CrossRef]
- Bultosa, G.; Taylor, J.R.N. Teff. In Encyclopedia of Grain Science; Wrigley, C., Corke, H., Walker, C., Eds.; Elsevier: London, UK, 2004; pp. 281–290. [Google Scholar]
- Hager, A.S.; Wolter, A.; Jacob, F.; Zannini, E.; Arendt, E.K. Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J. Cereal Sci. 2012, 56, 239–247. [Google Scholar] [CrossRef]
- World Watermelon Production by Country. Available online: https://www.atlasbig.com/en-us/countries-watermelon-production (accessed on 25 March 2023).
- El-Adawy, T.A.; Taha, K.M. Characteristics and Composition of Watermelon, Pumpkin, and Paprika Seed Oils and Flours. J. Agric. Food Chem. 2001, 49, 1253–1259. [Google Scholar] [CrossRef]
- Mahla, H.R.; Rathore, S.S.; Venkatesan, K.; Sharma, R. Analysis of fatty acid methyl esters and oxidative stability of seed purpose watermelon (Citrullus lanatus) genotypes for edible oil. J. Food Sci. Technol. 2018, 55, 1552–1561. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef]
- Manivannan, A.; Lee, E.-S.; Han, K.; Lee, H.-E.; Kim, D.-S. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules 2020, 25, 5258. [Google Scholar] [CrossRef]
- Conforti, F.D.; Johnson, J.M. Use of the Farinograph in Predicting Baking Quality of Unchlorinated and Chlorinated Flours. J. Food Qual. 1992, 15, 333–347. [Google Scholar] [CrossRef]
- Katyal, M.; Kaur, A.; Singh, N. Evaluation of pasting and dough rheological properties of composite flours made from flour varied in gluten strength. J. Food Sci. Technol. 2019, 56, 2700–2711. [Google Scholar] [CrossRef]
- Coleman, J.; Abaye, A.O.; Barbeau, W.; Thomason, W. The suitability of teff flour in bread, layer cakes, cookies and biscuits. Int. J. Food Sci. Nutr. 2013, 64, 877–881. [Google Scholar] [CrossRef]
- Anang, D.A.; Pobee, R.A.; Antwi, E.; Obeng, E.M.; Atter, A.; Ayittey, F.K.; Boateng, J.T. Nutritional, microbial and sensory attributes of bread fortified with defatted watermelon seed flour. Int. J. Food Sci. 2018, 53, 1468–1475. [Google Scholar] [CrossRef]
- Wani, A.A.; Sogi, D.S.; Singh, P.; Khatkar, B.S. Influence of watermelon seed protein concentrates on dough handling, textural and sensory properties of cookies. J. Food Sci. Technol. 2015, 52, 2139–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169407/nutrients (accessed on 15 February 2023).
- Salawu, S.O.; Bester, M.J.; Duodu, K.G. Phenolic composition and bioactive properties of cell wall preparations and whole grains of selected cereals and legumes. J. Food Biochem. 2013, 38, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Shumoy, H.; Raes, K. Antioxidant Potentials and Phenolic Composition of Tef Varieties: An Indigenous Ethiopian Cereal. Cereal Chem. 2016, 93, 465–470. [Google Scholar] [CrossRef]
- Kotaskova, E.; Sumczynski, D.; Mlcek, J.; Valasek, P. Determination of free and bound phenolics using HPLC-DAD, antioxidant activity and in vitro digestibility of Eragrostis tef. J. Food Composit. Anal. 2016, 46, 15–21. [Google Scholar] [CrossRef]
- Ravisankar, S.; Abegaz, K.; Awika, J.M. Structural profile of soluble and bound phenolic compounds in teff (Eragrostis tef) reveals abundance of distinctly different flavones in white and brown varieties. Food Chem. 2018, 263, 265–274. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar constituents from hydro-methanolic extract of watermelon (Citrullus lanatus) by means of accurate-mass spectrometry (HPLC–ESI–QTOF–MS). Food Res. Int. 2013, 51, 354–362. [Google Scholar] [CrossRef]
- Sorokina, M.; McCaffrey, K.S.; Deaton, E.E.; Ma, G.; Ordovas, J.M.; Perkins-Veazie, P.M.; Steinbeck, C.; Levi, A.; Parnell, L.D. A Catalog of Natural Products Occurring in Watermelon—Citrullus lanatus. Front. Nutr. 2021, 8, 729822. [Google Scholar] [CrossRef] [PubMed]
- Tabiri, B.; Agbenorhevi, J.K.; Wireko-Manu, F.D.; Ompouma, E.I. Watermelon Seeds as Food: Nutrient Composition, Phytochemicals and Antioxidant Activity. Int. J. Nutr. Food Sci. 2016, 5, 139–144. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Oghabei, M.; Prakash, J. Bioaccessible phenolics and flavonoids from wheat flour products subjected to different processing variables. Cereal Chem. 2019, 96, 1068–1078. [Google Scholar] [CrossRef]
- Lu, Y.; Luthria, D.; Fuerst, E.P.; Kiszonas, A.M.; Yu, L.; Morris, C.F. Effect of Processing on Phenolic Composition of Dough and Bread Fractions Made from Refined and Whole Wheat Flour of Three Wheat Varieties. J. Agric. Food Chem. 2014, 62, 10431–10436. [Google Scholar] [CrossRef]
- Gil, J.V.; Esteban-Munoz, A.; Fernandez-Espinar, M.T. Changes in the Polyphenolic Profile and Antioxidant Activity of Wheat Bread after Incorporating Quinoa Flour. Antioxidants 2022, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Lis, B.; Jedrejek, D.; Rywaniak, J.; Soluch, A.; Stochmal, A.; Olas, B. Flavonoid Preparations from Taraxacum officinale L. Fruits—A Phytochemical, Antioxidant and Hemostasis Studies. Molecules 2020, 25, 5402. [Google Scholar] [CrossRef]
- El-Alfy, T.S.; Ezzat, S.M.; Sleem, A.A. Chemical and biological study of the seeds of Eragrostis tef (Zucc.) Trotter. Nat. Prod. Res. 2012, 26, 619–629. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants – Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.-Y.; Xu, D.-P.; Li, H.-B. Dietary sources and bioactivities of melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Jedrejek, D.; Pawelec, S.; Piwowarczyk, R.; Pecio, Ł.; Stochmal, A. Identification and occurrence of phenylethanoid and iridoid glycosides in six Polish broomrapes (Orobanche spp. and Phelipanche spp., Orobanchaceae). Phytochemistry 2020, 170, 112189. [Google Scholar] [CrossRef]
- Forsido, S.F.; Rupasinghe, H.P.V.; Astatkie, T. Antioxidant capacity, total phenolics and nutritional content in selected ethiopian staple food ingredients. Int. J. Food Sci. Nutr. 2013, 64, 915–920. [Google Scholar] [CrossRef]
- Miladinović, B.; Ilić, K.; Stojanović, D.; Kostić, M.; Milutinović, M.; Branković, S.; Kitić, D. Antioxidant activity, total phenol and tannin content of different varieties of flours. Acta Med. Median. 2020, 59, 100–107. [Google Scholar] [CrossRef]
- Sedej, I.; Sakać, M.; Mandić, A.; Misan, A.; Tumbas, V.; Hadnadev, M. Assessment of antioxidant activity and rheological properties of wheat and buckwheat milling fractions. J. Cereal Sci. 2011, 54, 347–353. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A.; Stankowski, S.; Sobolewska, M.; Kępińska-Pacelik, J. Comparison of yield, chemical composition and farinograph properties of common and ancient wheat grains. Eur. Food Res. Technol. 2021, 247, 1525–1538. [Google Scholar] [CrossRef]
- Lacko-Bartošová, M.; Konvalina, P.; Lacko-Bartošová, L.; Štěrba, Z. Quality evaluation of emmer wheat genotypes based on rheological and Mixolab parameters. Czech J. Food Sci. 2019, 37, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Rohrlich, M.; Bruckner, G. Cereals; Parey: Berlin, Germany, 1966; Volume I. (In German) [Google Scholar]
- ISO 6491:2000P; Animal Feeding Stuffs—Determination of Phosphorus Content—Spectrometric Method. ANSI: Washington, DC, USA, 1998.
- ISO 6869:2000; Animal Feeding Stuffs—Determination of The Contents of Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc—Method Using Atomic Absorption Spectrometry. ANSI: Washington, DC, USA, 2000.
- AOAC 920.87-1920; Protein (Total) in Flour. AOAC: Rockville, MD, USA, 2001.
- Setyaningsih, W.; Saputro, I.E.; Barbero, G.F.; Palma, M.; Barroso, C.G. Determination of melatonin in rice (Oryza sativa) grains by pressurized liquid extraction. J. Agric. Food Chem. 2015, 63, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, A.; Montenero-San-Martin, B.; Haro, N.; Pozo, O.J. Nail Melatonin Content: A Suitable Non-Invasive Marker of Melatonin Production. Int. J. Mol. Sci. 2021, 22, 921. [Google Scholar] [CrossRef]
- Kontek, B.; Jedrejek, D.; Oleszek, W.; Olas, B. Antiradical and antioxidant activity in vitro of hops-derived extracts rich in bitter acids and xanthohumol. Ind. Crops Prod. 2021, 161, 113208. [Google Scholar] [CrossRef]
- Rahman, M.J.; de Camargo, A.; Shahidi, F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J. Func. Foods 2017, 35, 622–634. [Google Scholar] [CrossRef]
- PN-EN ISO 5530-1:2015-01E; Wheat Flour—Physical Characteristics of Doughs—Part 1: Determination of Water Absorption and Rheological Properties Using a Farinograph. Polish Committee for Standardization: Warsaw, Poland, 2015.
- Bultosa, G. Physicochemical Characteristics of Grain and Flour in 13 Tef [Eragrostis tef (Zucc.) Trotter] Grain Varieties. Res. J. Appl. Sci. 2007, 3, 2042–2051. [Google Scholar]
- Baye, K.; Mouquet-Rivier, C.; Icard-Verniere, C.; Picq, C.; Guyot, J.-P. Changes in mineral absorption inhibitors consequent to fermentation of Ethiopian injera: Implications for predicted iron bioavailability and bioaccessibility. Int. J. Food Sci. 2014, 49, 174–180. [Google Scholar] [CrossRef]
- Alemneh, S.T.; Emire, S.A.; Hitzmann, B.; Zettel, V. Comparative Study of Chemical Composition, Pasting, Thermal and Functional properties of Teff (Eragrostis tef) Flours Grown in Ethiopia and South Africa. Int. J. Food Prop. 2022, 25, 144–158. [Google Scholar] [CrossRef]
- USDA FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169747/nutrients (accessed on 15 February 2023).
- Rezig, L.; Chouaibi, M.; Meddeb, W.; Msaada, K.; Hamdi, S. Chemical composition and bioactive compounds of Cucurbitaceae seeds: Potential sources for new trends of plant oils. Process Saf. Environ. Prot. 2019, 127, 73–81. [Google Scholar] [CrossRef]
- Kausar, T.; Hassan, M.T.; Din, G.M. Utilization of watermelon seed flour as protein supplement in cookies. Pure Appl. Biol. 2020, 9, 202–206. [Google Scholar] [CrossRef]
- Eke, R.; Ejiofor, E.; Oyedemi, S.; Onoja, S.; Omeh, N. Evaluation of nutritional composition of Citrullus lanatus Linn. (watermelon) seed and biochemical assessment of the seed oil in rats. Food Biochem. 2021, 45, e13763. [Google Scholar] [CrossRef]
- Japu, J.A.; Ahmed, S.; Hossain, M.A.; Ahmed, T.; Ali, M.S. Dietary Composition and Functional Properties of Selected Watermelon Seeds Available in Bangladesh. SAARC J. Agric. 2021, 19, 233–243. [Google Scholar] [CrossRef]
- Gebru, Y.A.; Kim, D.-W.; Sbhatu, D.B.; Abraha, H.B.; Lee, J.W.; Choi, Y.B.; Kim, Y.-H.; Kim, M.-K.; Kim, K.-P. Comparative analysis of total phenol, total flavonoid and in vitro antioxidant capacity of white and brown teff (Eragrostis tef), and identification of individual compounds using UPLC-qTOF-MS. J. Food Meas. Charact. 2021, 15, 5392–5407. [Google Scholar] [CrossRef]
- Neglo, D.; Tettey, C.O.; Essuman, E.K.; Kortei, N.K.; Boakye, A.A.; Hunkpe, G.; Amarh, F.; Kwashie, P.; Devi, W.S. Comparative antioxidant and antimicrobial activities of the peels, rind, pulp and seeds of watermelon (Citrullus lanatus) fruit. Sci. Afr. 2021, 11, e00582. [Google Scholar] [CrossRef]
| Parameter | Teff | Watermelon Seeds | Watermelon Seed Pomace | |
|---|---|---|---|---|
| Macroelements | P (g·kg−1 dm a) | 5.40 ± 0.1 c | 6.05 ± 0.1 b | 7.50 ± 0.1 a |
| K (g·kg−1 dm) | 2.71 ± 0.1 c | 6.31 ± 0.0 a | 5.14 ± 0.1 b | |
| Ca (g·kg−1 dm) | 0.89 ± 0.0 a | 0.35 ± 0.0 c | 0.50 ± 0.0 b | |
| Mg (g·kg−1 dm) | 1.53 ± 0.0 b | 1.54 ± 0.0 b | 1.72 ± 0.0 a | |
| Na (g·kg−1 dm) | 0.08 ± 0.0 b | 0.10 ± 0.0 b | 0.44 ± 0.0 a | |
| Microelements | Fe (mg·kg−1 dm) | 60.30 ± 0.7 b | 28.70 ± 0.9 c | 124.20 ± 0.2 a |
| Zn (mg·kg−1 dm) | 22.90 ± 0.1 c | 25.20 ± 0.0 b | 27.50 ± 0.0 a | |
| Mn (mg·kg−1 dm) | 42.60 ± 0.4 a | 16.10 ± 0.2 b | 13.80 ± 0.3 c | |
| Cu (mg·kg−1 dm) | nd b | nd | nd | |
| Mo (mg·kg−1 dm) | 13.80 ± 0.7 b | 12.50 ± 0.7 b | 17.50 ± 0.3 a | |
| Toxic metals | Pb (mg·kg−1 dm) | nd | nd | nd |
| Cd (mg·kg−1 dm) | nd | nd | nd | |
| Crude protein (%) | 11.70 ± 0.1 c | 20.50 ± 0.1 b | 25.20 ± 0.1 a | |
| Total lipids (%) | 2.87 ± 0.0 c | 29.61 ± 0.5 a | 8.99 ± 0.1 b |
| No | RT (min) | UVmax (nm) | [M-H]−, m/z | MS/MS Fragments a | [M+H] +, m/z | MS/MS Fragments a | Identity | Presence in Sample b | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Teff Flour | Watermelon Seed Flour | Watermelon Seed Pomace Flour | ||||||||
| 1 | 5.12 | 255, 345 | 609 | 327, 357, 411 | 611 | 329, 431, 449 | luteolin di-hex d | + | nd | nd |
| 2 | 5.20 | 287, 325 | 355 | 175, 160, 193 | 379 c/357 | - | ferulic acid hex | nd | + | + |
| 3 | 5.22 | 255, 269, 348 | 609 | 327, 357, 411 | 611 | 329, 299, 353 | luteolin di-hex | +++ | nd | nd |
| 4 | 5.54 | 295sh, 325 | 401 | 101, 71, 161 | 425 c | - | unidentified | nd | trace | + |
| 5 | 5.78 | 269, 335 | 593 | 311, 341 | 595 | 313, 283, 397 | apigenin di-hex | + | nd | nd |
| 6 | 6.00 | 269, 348 | 447 | 357, 327, 297 | 449 | 299, 329 | luteolin C-hex | + | trace | + |
| 7 | 6.06 | 271, 335 | 623 | 341, 371 | 625 | 343, 367, 313 | methoxyluteolin di-hex | + | nd | nd |
| 8 | 6.19 | 256, 269, 350 | 447 | 327, 357, 297 | 449 | 329, 299, 413 | luteolin C-hex | +++ | + | + |
| 9 | 6.70 | 268, 336 | 431 | 311, 283, 341 | 433 | 313, 283, 397 | apigenin C-hex | + | ++ | +++ |
| 10 | 6.80 | 255, 269, 348 | 593 | 285 | 595 | 287, 449 | luteolin O-deoxyhex-hex | + | nd | nd |
| 11 | 6.99 | 255, 269, 348 | 447 | 447, 285 | 449 | 287 | luteolin O-hex | ++ | nd | nd |
| 12 | 7.11 | 264, 347 | 759 | 327, 357, 411 | 761 | 151, 329, 431 | luteolin O-syringyl-pentoside C-hex | +++ | nd | nd |
| 13 | 7.67 | 339 | 177 | 162 | 179 | 91, 79, 146 | hydroxy-methoxycinnamyl aldehyde | nd | ++ | + |
| 14 | 7.75 | 265, 341 | 447 | 285 | 449 | 287 | luteolin-7-O-glucoside # | nd | +++ | ++ |
| 15 | 7.82 | 269, 348 | 489 | 327, 299, 357 | 491 | 329, 299, 311 | luteolin C-acetylhex | + | nd | nd |
| 16 | 7.90 | 339 | 177 | 162, 134 | 179 | 91, 79, 119 | hydroxy-methoxycinnamyl aldehyde | nd | + | + |
| 17 | 8.01 | 255, 345 | 461 | 283, 446, 298 | 463 | 301 | methoxyluteolin O-hex | trace | trace | + |
| 18 | 8.91 | 277 | 339 | 263, 291, 327 | 341 | 137, 251 | unidentified | nd | + | trace |
| 19 | 9.40 | 255, 269, 347 | 285 | 133, 151, 175 | 287 | 287, 153, 135 | luteolin # | nd | +++ | +++ |
| 20 | 9.79 | 269, 345 | 551 | 473, 165, 503 | 575 c/553 | - | unidentified bi-flavonoid | nd | + | trace |
| 21 | 9.92 | 269, 345 | 551 | 165, 325, 195 | 575 c/553 | - | unidentified bi-flavonoid | nd | + | trace |
| 22 | 10.11 | 345 | 337 | 279, 307, 291 | 339 | 137, 219, 161 | unidentified | nd | +++ | ++ |
| 23 | 10.55 | 267, 337 | 269 | 117, 151, 149 | 271 | 271, 153, 119 | apigenin # | nd | + | +++ |
| 24 | 10.88 | 255, 269, 350 | 299 | 284, 256 | 301 | 286, 258, 301 | chrysoeriol # | nd | ++ | +++ |
| 25 | 11.11 | 315 | 383 | 163, 119, 145 | 407 c/385 | 147 | di-coumaroyl-glycerol | + | nd | nd |
| 26 | 11.37 | 320 | 413 | 163, 193, 145 | 437 c | 147 | coumaroyl-feruloyl-glycerol | + | nd | nd |
| 27 | 11.49 | 220, 345 | 515 | 219, 467, 485 | 539 c | 137, 427, 455 | unidentified | nd | + | trace |
| No | Compound | Content (mg Luteolin eq/kg of Plant Material) | ||
|---|---|---|---|---|
| Teff Flour (TF) | Watermelon Seed Flour (WSF) | Watermelon Seed Pomace Flour (DWSF) | ||
| 1 | luteolin di-hex | 3.03 ± 0.17 | nd | nd |
| 2 | luteolin di-hex | 70.86 ± 1.14 | nd | nd |
| 3 | apigenin di-hex | 8.12 ± 0.44 | nd | nd |
| 4 | luteolin C-hex | 6.66 ± 0.61 a | trace | 5.92 ± 0.37 |
| 5 | methoxyluteolin di-hex | 5.35 ± 0.70 | nd | nd |
| 6 | luteolin C-hex | 91.41 ± 1.01 c | 1.71 ± 0.29 a | 6.01 ± 0.30 b |
| 7 | apigenin C-hex | 7.53 ± 0.27 a | 18.63 ± 1.23 b | 102.94 ± 6.20 c |
| 8 | luteolin O-deoxyhex-hex | 11.16 ± 0.27 | nd | nd |
| 9 | luteolin O-hex | 10.90 ± 0.39 | nd | nd |
| 10 | luteolin O-syringyl-pentoside C-hex | 28.53 ± 0.73 | nd | nd |
| 11 | luteolin-7-O-glucoside | nd | 10.65 ± 0.57 a | 37.03 ± 2.14 b |
| 12 | luteolin C-acetylhex | 4.87 ± 0.16 | nd | nd |
| 13 | methoxyluteolin O-hex | trace | trace | 4.79 ± 0.50 |
| 14 | luteolin | nd | 42.51 ± 1.19 a | 372.07 ± 20.64 b |
| 15 | unidentified flavonoid | nd | 1.57 ± 0.21 | trace |
| 16 | unidentified flavonoid | nd | 2.27 ± 0.29 | trace |
| 17 | apigenin | nd | 6.07 ± 0.54 a | 91.17 ± 4.89 b |
| 18 | chrysoeriol | nd | 9.46 ± 0.45 a | 101.14 ± 5.89 b |
| total flavonoids (mg luteolin eq/kg) | 243.56 ± 3.27 b | 92.87 ± 3.66 a | 721.09 ± 39.64 c | |
| total phenolic content (mg GAE/g) | 0.51 ± 0.03 a | 1.01 ± 0.03 b | 1.67 ± 0.03 c | |
| melatonin (µg/kg) | 3.50 ± 0.24 a | 11.29 ± 0.48 b | 63.33 ± 1.28 c | |
| No | Compound | RT (min) | MS Signals *, m/z | Presence in Sample ** | |||
|---|---|---|---|---|---|---|---|
| [M●] + | Characteristic Fragment Ions | Teff Flour | Watermelon Seed Flour | Watermelon Seed Pomace Flour | |||
| 1 | unidentified | 13.31 | 164(?) | 29, 31, 57, 73, 43 | nd | nd | ++ |
| 2 | unidentified | 14.13 | 164(?) | 57, 29, 31, 73, 42 | +++ | nd | nd |
| 3 | Palmitic acid | 23.54 | 256 | 43, 73, 60, 41, 57 | nd | + | nd |
| 4 | Linoleic acid | 27.51 | 280 | 67, 81, 82, 95, 55 | nd | +++ | nd |
| 5 | (Z)-9-Octadecenamide | 30.33 | 281 | 59, 72, 55, 41, 43 | trace | trace | + |
| 6 | unidentified | 35.08 | 410(?) | 117, 131, 67, 41, 81 | trace | nd | ++ |
| 7 | unidentified | 35.40 | 410(?) | 67, 55, 81, 117, 95 | nd | ++ | nd |
| 8 | Squalene | 37.00 | 410 | 69, 81, 41, 136, 137 | nd | + | + |
| 9 | β-Tocopherol | 40.06 | 416 | 151, 43, 191, 55, 57 | + | + | + |
| 10 | Stigmasterol | 42.65 | 412 | 55, 43, 81, 69, 83 | nd | + | + |
| 11 | β-Sitosterol | 43.33 | 414 | 43, 55, 41, 57, 107 | ++ | nd | nd |
| 12 | Isomultiflorenon | 43.74 | 424 | 205, 257, 245, 121, 119 | nd | + | + |
| 13 | β-Amyrin | 44.22 | 426 | 218, 203, 219, 189, 95 | + | nd | nd |
| 14 | Lupeol | 44.23 | 426 | 68, 55, 67, 81, 95 | nd | trace | trace |
| Sample | IC50 (mg of Plant Material/mL) | ||
|---|---|---|---|
| ABTS Assay | DPPH Assay | FCA Assay | |
| Teff flour (TF) | 156.72 ± 0.47 d (6.62 ± 0.02) # | 73.93 ± 0.48 c (3.04 ± 0.03) | 155.58 ± 1.70 d (5.16 ± 0.03) |
| Watermelon seed flour (WSF) | 116.66 ± 0.61 c (15.73 ± 0.38) | 107.68 ± 1.49 d (15.38 ± 0.21) | 95.25 ± 4.47 b (11.55 ± 0.54) |
| Watermelon seed pomace flour (DWSF) | 78.60 ± 1.18 b (4.52 ± 0.08) | 45.97 ± 0.63 b (2.68 ± 0.04) | 114.43 ± 7.16 c (6.35 ± 0.16) |
| Control positive (Trolox * or EDTA **) | 0.14 ± 0.00 *a | 0.10 ± 0.00 *a | 0.06 ± 0.00 **a |
| Sample | Farinograph Parameter | Flour Quality | ||||
|---|---|---|---|---|---|---|
| Water Absorption (%) | Development Time (min) | Stability Time (min) | Degree of Softening (after 10 min, FU) | Farinograph Quality Number (mm) | ||
| WF a + 10% TF b | 57.3 ± 0.2 bc | 4.6 ± 0.1 ab | 7.5 ± 0.1 bc | 37.0 ± 0.0 e | 88 ± 0.1 b | strong |
| WF + 20% TF | 56.9 ± 0.2 c | 4.5 ± 0.1 ab | 5.9 ± 0.0 d | 50.0 ± 0.0 b | 81 ± 0.7 c | strong |
| WF + 30% TF | 56.6 ± 0.2 cd | 3.9 ± 0.5 bc | 4.2 ± 0.1 e | 71.5 ± 0.7 a | 60 ± 0.0 e | medium |
| WF + 10% WSF c | 56.8 ± 0.2 cd | 5.5 ± 0.5 ab | 8.5 ± 0.1 ab | 28.5 ± 0.7 f | 82 ± 0.0 c | strong |
| WF + 20% WSF | 56.1 ± 0.0 de | 4.3 ± 0.1 abc | 6.1 ± 0.0 d | 53.5 ± 0.7 b | 73 ± 0.7 d | medium |
| WF + 30% WSF | 56.6 ± 0.2 cd | 3.9 ± 0.6 bc | 4.3 ± 0.1 e | 70.0 ± 0.7 a | 63 ± 0.1 e | medium |
| WF + 10% DWSF d | 57.2 ± 0.0 bc | 6.0 ± 0.7 a | 7.2 ± 0.1 c | 37.5 ± 0.7 e | 77 ± 0.0 d | strong |
| WF + 20% DWSF | 57.9 ± 0.4 ab | 5.5 ± 0.7 ab | 6.9 ± 0.1 cd | 40.5 ± 0.7 d | 78 ± 0.1 cd | strong |
| WF + 30% DWSF | 58.7 ± 0.0 a | 4.9 ± 0.7 ab | 8.9 ± 0.7 a | 44.0 ± 0.7 c | 100 ± 0.0 a | strong |
| WF (control) | 55.4 ± 0.3 e | 2.6 ± 0.1 c | 6.8 ± 0.2 cd | 51.5 ± 0.7 b | 62 ± 0.0 e | medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaroszewska, A.; Jedrejek, D.; Sobolewska, M.; Kowalska, I.; Dzięcioł, M. Mineral, Nutritional, and Phytochemical Composition and Baking Properties of Teff and Watermelon Seed Flours. Molecules 2023, 28, 3255. https://doi.org/10.3390/molecules28073255
Jaroszewska A, Jedrejek D, Sobolewska M, Kowalska I, Dzięcioł M. Mineral, Nutritional, and Phytochemical Composition and Baking Properties of Teff and Watermelon Seed Flours. Molecules. 2023; 28(7):3255. https://doi.org/10.3390/molecules28073255
Chicago/Turabian StyleJaroszewska, Anna, Dariusz Jedrejek, Magdalena Sobolewska, Iwona Kowalska, and Małgorzata Dzięcioł. 2023. "Mineral, Nutritional, and Phytochemical Composition and Baking Properties of Teff and Watermelon Seed Flours" Molecules 28, no. 7: 3255. https://doi.org/10.3390/molecules28073255
APA StyleJaroszewska, A., Jedrejek, D., Sobolewska, M., Kowalska, I., & Dzięcioł, M. (2023). Mineral, Nutritional, and Phytochemical Composition and Baking Properties of Teff and Watermelon Seed Flours. Molecules, 28(7), 3255. https://doi.org/10.3390/molecules28073255