Simple Co-Precipitation of Iron Minerals for the Removal of Phenylarsonic Acid: Insights into the Adsorption Performance and Mechanism
Abstract
:1. Introduction
2. Results
2.1. Characterizations of Ferrihydrite, Hematite, and Goethite
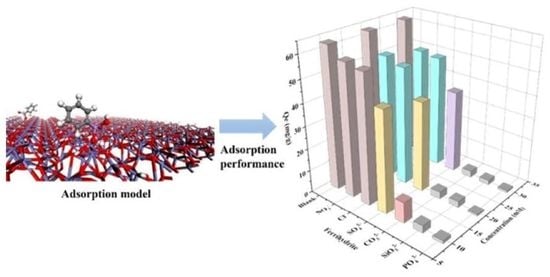
2.2. PAA Adsorption Performance of Ferrihydrite, Hematite, and Goethite
2.3. Effect of Main Parameters on the Adsorption of PAA
2.4. Mechanism of Adsorption
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Ferrihydrite, Goethite, and Hematite
3.3. Adsorption Experiments
3.3.1. Isothermal Adsorption Experiment
3.3.2. Adsorption Kinetics Experiment
3.3.3. Influencing Factor Testing
3.4. Characterization and Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and Human Health Effects: A Review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Tu, Y.; Wang, H.; Wang, Z.; Li, Y.; Chai, L.; Zhang, W.; Lin, Z. Environmental Behavior, Human Health Effect, and Pollution Control of Heavy Metal(Loid)s toward Full Life Cycle Processes. Eco-Environ. Health 2022, 1, 229–243. [Google Scholar] [CrossRef]
- de Souza, T.D.; Borges, A.C.; Braga, A.F.; Veloso, R.W.; de Matos, A.T. Phytoremediation of Arsenic-Contaminated Water by Lemna Valdiviana: An Optimization Study. Chemosphere 2019, 234, 402–408. [Google Scholar] [CrossRef]
- Hare, V.; Chowdhary, P.; Kumar, B.; Sharma, D.C.; Baghel, V.S. Arsenic Toxicity and Its Remediation Strategies for Fighting the Environmental Threat. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagava, R.N., Chowdhary, P., Eds.; Springer Singapore: Singapore, 2019; pp. 143–170. ISBN 978-981-10-8668-7. [Google Scholar]
- Liu, Q.; Lu, X.; Peng, H.; Popowich, A.; Tao, J.; Uppal, J.S.; Yan, X.; Boe, D.; Le, X.C. Speciation of Arsenic—A Review of Phenylarsenicals and Related Arsenic Metabolites. Trends Analyt. Chem. 2018, 104, 171–182. [Google Scholar] [CrossRef]
- Vega, L.; Styblo, M.; Patterson, R.; Cullen, W.; Wang, C.; Germolec, D. Differential Effects of Trivalent and Pentavalent Arsenicals on Cell Proliferation and Cytokine Secretion in Normal Human Epidermal Keratinocytes. Toxicol. Appl. Pharmacol. 2001, 172, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhao, Z.; Liang, Z.; Liu, J.; Shi, W.; Cui, F. Efficient Degradation of P-Arsanilic Acid with Arsenic Adsorption by Magnetic CuO-Fe3O4 Nanoparticles under Visible Light Irradiation. Chem. Eng. J. 2018, 334, 1527–1536. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Jin, W.; Hu, Q.; Zhao, Y. Adsorption Behavior of Arsenicals on MIL-101(Fe): The Role of Arsenic Chemical Structures. J. Colloid Interface Sci. 2019, 554, 692–704. [Google Scholar] [CrossRef]
- Oya-Ohta, Y.; Kaise, T.; Ochi, T. Induction of Chromosomal Aberrations in Cultured Human Fibroblasts by Inorganic and Organic Arsenic Compounds and the Different Roles of Glutathione in Such Induction. Mutat. Res. 1996, 357, 123–129. [Google Scholar] [CrossRef]
- Peng, X.; Chen, L.; Liu, S.; Hu, L.; Zhang, J.; Wang, A.; Yu, X.; Yan, Z. Insights into the Interfacial Interaction Mechanisms of P-Arsanilic Acid Adsorption on Ionic Liquid Modified Porous Cellulose. J. Environ. Chem. Eng. 2021, 9, 105225. [Google Scholar] [CrossRef]
- Kroening, K.K.; Solivio, M.J.V.; García-López, M.; Puga, A.; Caruso, J.A. Cytotoxicity of Arsenic-Containing Chemical Warfare Agent Degradation Products with Metallomic Approaches for Metabolite Analysis. Metallomics 2009, 1, 59–66. [Google Scholar] [CrossRef]
- Cai, C.; Zhao, M.; Yu, Z.; Rong, H.; Zhang, C. Utilization of Nanomaterials for In-Situ Remediation of Heavy Metal(Loid) Contaminated Sediments: A Review. Sci. Total Environ. 2019, 662, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Y.; Li, H.; Ye, M.; Qin, W.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Electrodeposition of Hierarchically Amorphous FeOOH Nanosheets on Carbonized Bamboo as an Efficient Filter Membrane for As(III) Removal. Chem. Eng. J. 2020, 392, 123773. [Google Scholar] [CrossRef]
- Assaad, N.; Sabeh, G.; Hmadeh, M. Defect Control in Zr-Based Metal–Organic Framework Nanoparticles for Arsenic Removal from Water. ACS Appl. Nano Mater. 2020, 3, 8997–9008. [Google Scholar] [CrossRef]
- Weerasundara, L.; Ok, Y.-S.; Bundschuh, J. Selective Removal of Arsenic in Water: A Critical Review. Environ. Pollut. 2021, 268, 115668. [Google Scholar] [CrossRef] [PubMed]
- Rahidul Hassan, H. A Review on Different Arsenic Removal Techniques Used for Decontamination of Drinking Water. Env Pollut. Bioavail. 2023, 35, 2165964. [Google Scholar] [CrossRef]
- Fu, X.; Song, X.; Zheng, Q.; Liu, C.; Li, K.; Luo, Q.; Chen, J.; Wang, Z.; Luo, J. Frontier Materials for Adsorption of Antimony and Arsenic in Aqueous Environments: A Review. Int. J. Environ. Res. Public Health 2022, 19, 10824. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Iron Oxide and Its Modified Forms as an Adsorbent for Arsenic Removal: A Comprehensive Recent Advancement. Process Saf. Environ. Prot. 2017, 111, 592–626. [Google Scholar] [CrossRef]
- Ishizaki, M.; Yanaoka, T.; Nakamura, M.; Hakuta, T.; Ueno, S.; Komuro, M.; Shibata, M.; Kitamura, T.; Honda, A.; Doy, M.; et al. Detection of Bis(Diphenylarsine)Oxide, Diphenylarsinic Acid and Phenylarsonic Acid, Compounds Probably Derived from Chemical Warfare Agents, in Drinking Well Water. J. Health Sci. 2005, 51, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Cheng, H. Adsorption and Desorption of Phenylarsonic Acid Compounds on Metal Oxide and Hydroxide, and Clay Minerals. Sci. Total Environ. 2021, 757, 143765. [Google Scholar] [CrossRef]
- Sparks, D.L. Toxic Metals in the Environment: The Role of Surfaces. Elements 2005, 1, 193–197. [Google Scholar] [CrossRef]
- Ginder-Vogel, M.; Landrot, G.; Fischel, J.S.; Sparks, D.L. Quantification of Rapid Environmental Redox Processes with Quick-Scanning X-Ray Absorption Spectroscopy (Q-XAS). Proc. Natl. Acad. Sci. USA 2009, 106, 16124–16128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebecker, M.; Li, W.; Khalid, S.; Sparks, D. Real-Time QEXAFS Spectroscopy Measures Rapid Precipitate Formation at the Mineral–Water Interface. Nat. Commun. 2014, 5, 5003. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Mulligan, C.N. Speciation and Surface Structure of Inorganic Arsenic in Solid Phases: A Review. Environ. Int. 2008, 34, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Carabante, I.; Grahn, M.; Holmgren, A.; Kumpiene, J.; Hedlund, J. Adsorption of As (V) on Iron Oxide Nanoparticle Films Studied by in Situ ATR-FTIR Spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 106–113. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Li, K. One-Step Synthesis of Mesoporous Two-Line Ferrihydrite for Effective Elimination of Arsenic Contaminants from Natural Water. Dalton Trans. 2011, 40, 2062–2066. [Google Scholar] [CrossRef]
- Tanaka, M.; Togo, Y.S.; Yamaguchi, N.; Takahashi, Y. An EXAFS Study on the Adsorption Structure of Phenyl-Substituted Organoarsenic Compounds on Ferrihydrite. J. Colloid Interface Sci. 2014, 415, 13–17. [Google Scholar] [CrossRef]
- Yan, L.; Chan, T.; Jing, C. Arsenic Adsorption on Hematite Facets: Spectroscopy and DFT Study. Environ. Sci. Nano. 2020, 7, 3927–3939. [Google Scholar] [CrossRef]
- Chen, P.; Song, D.; Zhang, X.; Xie, Q.; Zhou, Y.; Liu, H.; Xu, L.; Chen, T.; Rosso, K.M. Understanding Competitive Phosphate and Silicate Adsorption on Goethite by Connecting Batch Experiments with Density Functional Theory Calculations. Environ. Sci. Technol. 2022, 56, 823–834. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, X.; Huang, X.; Wan, S.; An, X.; Jia, F.; Zhang, L. Insights into the Facet-Dependent Adsorption of Phenylarsonic Acid on Hematite Nanocrystals. Environ. Sci. Nano. 2019, 6, 3280–3291. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Xia, S. Ferrihydrite/Ultrasound Activated Peroxymonosulfate for Humic Acid Removal. Turk. J. Chem. 2022, 46, 835–848. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Effects of Adsorbed Arsenate on the Rate of Transformation of 2-Line Ferrihydrite at PH 10. Environ. Sci. Technol. 2011, 45, 5557–5563. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm Models for Adsorption of Heavy Metals from Water—A Review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, L.; Ma, Q.; Liu, Y.; Li, J.; Li, Z.; Li, S.; Zhang, T. Ball Milling Enhanced Cr(VI) Removal of Zero-Valent Iron Biochar Composites: Functional Groups Response and Dominant Reduction Species. Chemosphere 2023, 311, 137174. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Ma, Y.; Wan, J.; Wang, Y. Removal of Gentian Violet and Rhodamine B Using Banyan Aerial Roots after Modification and Mechanism Studies of Differential Adsorption Behaviors. Environ. Sci. Pollut. Res. 2020, 27, 9152–9166. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Wang, L.; Pan, L.; Zhang, X.; Zou, J.-J. Rational Design, Synthesis, Adsorption Principles and Applications of Metal Oxide Adsorbents: A Review. Nanoscale 2020, 12, 4790–4815. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Buthiyappan, A. Adsorption of Arsenic Using Chitosan Magnetic Graphene Oxide Nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef]
- Wang, G.; Sun, T.; Sun, Z.; Hu, X. Preparation of Copper Based Metal Organic Framework Materials and Its Effective Adsorptive Removal of Ceftazidime from Aqueous Solutions. Appl. Surf. Sci. 2020, 532, 147411. [Google Scholar] [CrossRef]
- Pan, T.; Liu, H.; Jiang, M.; Li, J.; Liu, W.; Jiao, Q.; Zhang, T. New Insights into the Adsorption Behavior of Thiacloprid at the Microfibers/Water Interface: Role of Humic Acid. Chemosphere 2023, 311, 136938. [Google Scholar] [CrossRef]
- Ofomaja, A.E. Intraparticle Diffusion Process for Lead(II) Biosorption onto Mansonia Wood Sawdust. Bioresour. Technol. 2010, 101, 5868–5876. [Google Scholar] [CrossRef]
- Ge, X.; Ma, Y.; Song, X.; Wang, G.; Zhang, H.; Zhang, Y.; Zhao, H. β-FeOOH Nanorods/Carbon Foam-Based Hierarchically Porous Monolith for Highly Effective Arsenic Removal. ACS Appl. Mater. Interfaces 2017, 9, 13480–13490. [Google Scholar] [CrossRef]
- Yu, X.; Wei, Y.; Liu, C.; Ma, J.; Liu, H.; Wei, S.; Deng, W.; Xiang, J.; Luo, S. Ultrafast and Deep Removal of Arsenic in High-Concentration Wastewater: A Superior Bulk Adsorbent of Porous Fe2O3 Nanocubes-Impregnated Graphene Aerogel. Chemosphere 2019, 222, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Huan, Z.; Zhang, J.; Li, J.; Li, Z.; Guo, P.; Zhu, Y.; Zhang, T. CTAB-Functionalized δ-FeOOH for the Simultaneous Removal of Arsenate and Phenylarsonic Acid in Phenylarsenic Chemical Warfare. Chemosphere 2022, 292, 133373. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Dong, H.; Ma, J.; Jiang, L. Removal of Arsenic from Water: Effects of Competing Anions on As(III) Removal in KMnO4–Fe(II) Process. Water Res. 2009, 43, 3891–3899. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, J.; Ding, Z.; Li, N.; Fu, F.; Tang, B. Cr(vi) Removal by Mesoporous FeOOH Polymorphs: Performance and Mechanism. RSC Adv. 2016, 6, 82118–82130. [Google Scholar] [CrossRef]
- Guo, T.; Wang, K.; Zhang, G.; Wu, X. A Novel α-Fe2O3@g-C3N4 Catalyst: Synthesis Derived from Fe-Based MOF and Its Superior Photo-Fenton Performance. Appl. Surf. Sci. 2019, 469, 331–339. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-Examination of Characteristic FTIR Spectrum of Secondary Layer in Bilayer Oleic Acid-Coated Fe3O4 Nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Rahimi, S.; Moattari, R.M.; Rajabi, L.; Derakhshan, A.A.; Keyhani, M. Iron Oxide/Hydroxide (α,γ-FeOOH) Nanoparticles as High Potential Adsorbents for Lead Removal from Polluted Aquatic Media. J. Ind. Eng. Chem. 2015, 23, 33–43. [Google Scholar] [CrossRef]





| Adsorbent | Langmuir | Freundlich | Redlich–Peterson | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | kL | R2 | kF | n | R2 | kR | α | β | R2 | |
| Ferrihydrite | 62.21 | 1.63 | 0.992 | 35.84 | 0.19 | 0.981 | 155 | 3.11 | 0.92 | 0.999 |
| Goethite | 22.99 | 0.13 | 0.996 | 6.21 | 0.314 | 0.982 | 2.14 | 0.036 | 1.24 | 0.999 |
| Hematite | 35.58 | 0.072 | 0.999 | 5.36 | 0.440 | 0.992 | 2.47 | 0.061 | 1.031 | 0.999 |
| Adsorbent | Pseudo First Order | Pseudo Second Order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe/(mg/g) | k1 | R2 | Qe/(mg/g) | k2 | R2 | α | β | R2 | |
| Ferrihydrite | 31.91 | 0.020 | 0.93 | 34.26 | 0.00085 | 0.98 | 3.87 | 0.19 | 0.99 |
| Goethite | 7.70 | 0.017 | 0.98 | 8.39 | 0.00267 | 0.99 | 0.48 | 0.70 | 0.95 |
| Hematite | 10.05 | 0.057 | 0.89 | 10.75 | 0.00725 | 0.96 | 6.78 | 0.75 | 0.99 |
| Adsorbent | Stage I | Stage II | Stage III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1d | C1 | R2 | k2d | C2 | R2 | k3d | C3 | R2 | |
| Ferrihydrite | 2.60 | 1.03 | 0.98 | 0.71 | 17.23 | 0.99 | 0.12 | 30.10 | 0.98 |
| Goethite | 0.70 | −0.28 | 0.98 | 0.19 | 3.88 | 0.99 | 0.02 | 7.52 | 0.97 |
| Hematite | 1.26 | 0.57 | 0.93 | 0.14 | 7.23 | 0.99 | 0.08 | 8.70 | 0.96- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Hu, C.; Yang, Z.; Guo, S.; Zhang, T.; Li, S. Simple Co-Precipitation of Iron Minerals for the Removal of Phenylarsonic Acid: Insights into the Adsorption Performance and Mechanism. Molecules 2023, 28, 3448. https://doi.org/10.3390/molecules28083448
Wang L, Hu C, Yang Z, Guo S, Zhang T, Li S. Simple Co-Precipitation of Iron Minerals for the Removal of Phenylarsonic Acid: Insights into the Adsorption Performance and Mechanism. Molecules. 2023; 28(8):3448. https://doi.org/10.3390/molecules28083448
Chicago/Turabian StyleWang, Lili, Changchao Hu, Ze Yang, Songding Guo, Tingting Zhang, and Shangyi Li. 2023. "Simple Co-Precipitation of Iron Minerals for the Removal of Phenylarsonic Acid: Insights into the Adsorption Performance and Mechanism" Molecules 28, no. 8: 3448. https://doi.org/10.3390/molecules28083448
APA StyleWang, L., Hu, C., Yang, Z., Guo, S., Zhang, T., & Li, S. (2023). Simple Co-Precipitation of Iron Minerals for the Removal of Phenylarsonic Acid: Insights into the Adsorption Performance and Mechanism. Molecules, 28(8), 3448. https://doi.org/10.3390/molecules28083448