Mechanically Stimulated Solid-State Interaction of Platinum Tetrachloride with Sodium β-Diketonates
Abstract
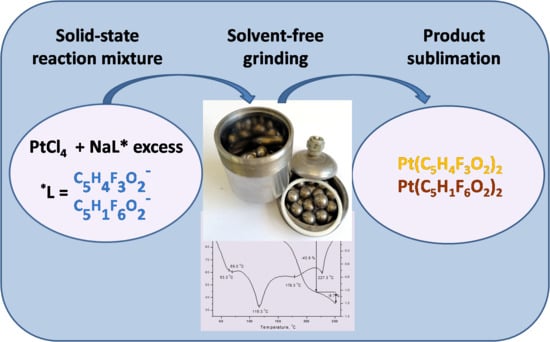
:1. Introduction
2. Results
3. Experimental
3.1. Materials and Methods
3.2. Synthesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Serp, P.; Kalck, P.; Feurer, R. Chemical Vapor Deposition Methods for the Controlled Preparation of Supported Catalytic Materials. Chem. Rev. 2002, 102, 3085–3128. [Google Scholar] [CrossRef]
- Hierso, J.-C.; Feurer, R.; Kalck, P. Platinum, palladium and rhodium complexes as volatile precursors for depositing materials. Coord. Chem. Rev. 1998, 178–180, 1811–1834. [Google Scholar] [CrossRef]
- Thurier, C.; Doppelt, P. Platinum OMCVD processes and precursor chemistry. Coord. Chem. Rev. 2008, 252, 155–189. [Google Scholar] [CrossRef]
- Karakovskaya, K.I.; Dorovskikh, S.I.; Vikulova, E.S.; Ilyin, I.Y.; Zherikova, K.V.; Basova, T.V.; Morozova, N.B. Volatile Iridium and Platinum MOCVD Precursors: Chemistry, Thermal Properties, Materials and Prospects for Their Application in Medicine. Coatings 2021, 11, 78. [Google Scholar] [CrossRef]
- Haque, A.; El Moll, H.; Alenezi, K.M. Functional Materials Based on Cyclometalated Platinum(II) β-Diketonate Complexes: A Review of Structure–Property Relationships and Applications. Materials 2021, 14, 4236. [Google Scholar] [CrossRef] [PubMed]
- Md. Raza, K.; Mitra, K.; Shettar, A.; Basu, U.; Kondaiah, P.; Chakravarty, A.R. Photoactive platinum(II) β-diketonates as dual action anticancer agents. Dalton Trans. 2016, 45, 13234–13243. [Google Scholar] [CrossRef]
- Spiegel, R.J. Platinum and fuel cells. Transp. Res. Part D 2004, 9, 357–371. [Google Scholar] [CrossRef]
- Okeya, S.; Kawaguchi, S. The bis(β-diketonato) platinum(II) complexes. In Inorganic Syntheses; Busch, D.H., Ed.; John Wiley & Sons: New York, NY, USA; Chichester, UK; Brisbcrne, Australia; Toronto, ON, Canada, 1980; Volume 20, pp. 65–69. [Google Scholar]
- Igumenov, I.K.; Isakova, V.G.; Morozova, N.B.; Shipachev, V.A. Method for producing tris-beta-diketonates of rare platinum metals. Patent RU 2 105 719, 27 February 1998. Available online: https://patenton.ru/patent/RU2105719C1.
- Dwyer, F.P.; Sargeson, A. Tris-acetylacetone-osmium(III). J. Am. Chem. Soc. 1955, 77, 1285. [Google Scholar] [CrossRef]
- Zherikova, K.V.; Mikheev, A.N.; Zharkova, G.I.; Morozova, N.B.; Igumenov, I.K.; Arzhannikov, A.V.; Tumm, M.K.A. Synthesis of ruthenium(III) and rhodium(III) tris-acetylacetonates and palladium(II) bis-ketoiminate using microwave heating. Russ. Chem. Bull. 2012, 61, 2236–2242. [Google Scholar] [CrossRef]
- Makhaev, V.; Petrova, L. Solid-phase synthesis of platinum group metal β-diketonates. Inorg. Chim. Acta 2021, 518, 120231. [Google Scholar] [CrossRef]
- Beyer, M.K.; Clausen-Schaumann, H. Mechanochemistry: The Mechanical Activation of Covalent Bonds. Chem. Rev. 2005, 105, 2921–2948. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 2006, 75, 177–189. [Google Scholar] [CrossRef]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- André, V.; Duarte, M.T.; Gomes, C.S.B.; Sarraguça, M.C. Mechanochemistry in Portugal—A Step towards Sustainable Chemical Synthesis. Molecules 2022, 27, 241. [Google Scholar] [CrossRef] [PubMed]
- Gomollón-Bel, F. Ten Chemical Innovations That Will Change Our World: IUPAC identifies emerging technologies in Chemistry with potential to make our planet more sustainable. Chem. Int. 2019, 41, 12–17. [Google Scholar] [CrossRef]
- Hernandez, J.G.; Halasz, I.; Crawford, D.E.; Krupicka, M.; Balaz, M.; Andre, V.; Vella-Zarb, L.; Niidu, A.; Garcia, F.; Maini, L.; et al. European Research in Focus: Mechanochemistry for Sustainable Industry (COST Action MechSustInd). Eur. J. Org. Chem. 2020, 2020, 8–9. [Google Scholar] [CrossRef]
- Beillard, A.; Bantreil, X.; Mếtro, T.-X.; Martinez, J.; Lamaty, F. Alternative Technologies That Facilitate Access to Discrete Metal Complexes. Chem. Rev. 2019, 119, 7529–7609. [Google Scholar] [CrossRef] [PubMed]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diff. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Batsanov, S.S.; Ruchkin, E.D. On the isomerism of mixed halogenides of platinum. Russ. J. Inorg. Chem. 1965, 10, 1415–1417. [Google Scholar]
- Krebs, B.; Brendel, C.; Schäfer, H. Neue Untersuchungen an α-Platindichlorid Darstellung und Struktur. Z. Für Anorg. Und Allg. Chem. 1988, 561, 119–131. [Google Scholar] [CrossRef]
- Butyagin, P.Y. Problems in mechanochemistry and prospects for its development. Russ. Chem. Rev. 1994, 63, 965–976. [Google Scholar] [CrossRef]
- Borisov, A.P.; Petrova, L.A.; Karpova, T.P.; Makhaev, V.D. The Solid-Phase Synthesis of Chromium p-Diketonates upon Mechanical Activation. Russ. J. Inorg. Chem. 1996, 41, 394–399. [Google Scholar]
- Pilbrow, M.F. Crystal structure of Platinum Tetrachloride. J. Chem. Soc. Chem. Commun. 1972, 270–271. [Google Scholar] [CrossRef]
- Borisov, A.P.; Petrova, L.A.; Makhaev, V. D Mechanochemical synthesis of organometallic compounds. In Proceedings of the 1st International Conference on Mechanochemistry INCOME’93; Tkacova, K., Ed.; Cambridge Interscience Publishing: Cambridge, UK, 1993; Volume 2, pp. 60–62. [Google Scholar]
- Makhaev, V.D.; Borisov, A.P. Mechanosynthesis of metal cyclopentadienyl complexes. In Mechanochemical Synthesis in Inorganic Chemistry; Boldyrev, V.V., Ed.; Siberian branch: Novosibirsk, Russia, 1991; pp. 165–168. (In Russian) [Google Scholar]
- Borisov, A.P.; Petrova, L.A. Solid-state mechanochemical synthesis of ferrocene. J. Organomet. Chem. 1999, 590, 222–226. [Google Scholar] [CrossRef]
- Borisov, A.P.; Makhaev, V.D.; Usyatinskii, A.Y.; Bregadze, V.I. Solid-state mechanochemical synthesis of cobalt(III), iron(III), and chromium(III) bisdicarbollyl complexes. Russ. Chem. Bull. 1993, 42, 1637–1639. [Google Scholar] [CrossRef]
- Makhaev, V.D.; Petrova, L.A. Mechanochemical Synthesis of vanadium(III) β-Diketonates. Russ. J. Gen. Chem. 2017, 87, 1105–1109. [Google Scholar] [CrossRef]
- Makhaev, V.D.; Petrova, L.A.; Alferov, K.A.; Belov, G.P. Mechanochemical Synthesis of Chromium Tris(2-ethylhexanoate) and Evaluation of Its Catalytic Activity in the Reaction of Ethylene Trimerization. Russ. J. Appl. Chem. 2013, 86, 1819–1824. [Google Scholar] [CrossRef]
- Petrova, L.A.; Makhaev, V.D. Mechanically activated solid-phase synthesis of copper(II), zinc(II), and cadmium(II) diethyldithiocarbamates. Russ. J. Inorg. Chem. 2007, 52, 865–870. [Google Scholar] [CrossRef]
- Makhaev, V.D.; Borisov, A.P.; Aleshin, V.V.; Petrova, L.A. Self-propagating synthesis of chromium acetylacetonate. Russ. Chem. Bull. 1995, 44, 1111–1113. [Google Scholar] [CrossRef]
- Ying, P.; Yu, J.; Su, W. Liquid-Assisted Grinding Mechanochemistry in the Synthesis of Pharmaceuticals. Adv. Synth. Catal. 2021, 363, 1159–1462. [Google Scholar] [CrossRef]
- Makhaev, V.D.; Borisov, A.P.; Aleshin, V.V.; Petrova, L.A.; Zuev, B.M. Self-Propagating Synthesis of Iron(III) Acetylacetonate after Mechanical Activation of the System FeCI3-NaC5H7O2. Int. J. Self-Propagating High-Temp. Synth. 2000, 9, 297–306, (and references therein). [Google Scholar]
- Avvakumov, E.G. Mekhanicheskie Metody Aktivatsii Khimicheskikh Protsessov (Mechanical Methods of Activation of Chemical Processes); Nauka: Novosibirsk, Russia, 1986. (In Russian) [Google Scholar]
- Petrova, L.A.; Borisov, A.P.; Aleshin, V.V.; Makhaev, V.D. Solid-Phase Synthesis of Copper(II) β-Diketonates upon Mechanical Activation. Russ. J. Inorg. Chem. 2001, 46, 1501–1506. [Google Scholar]
- Gómez-Benítez, V.; Germán-Acacio, J.M.; Morales-Morales, D. Mechanochemistry a Promising Tool on the Synthesis of Organometallic Pincer Compounds. Current State and Future Perspectives. Curr. Org. Chem. 2022, 26, 438–443. [Google Scholar] [CrossRef]
- Zharkova, G.I.; Igumenov, I.K.; Tkachev, S.V.; Zemskov, S.V. Platinum(II) β-Diketonates. Koord. Khimiya. 1982, 8, 74–81. ISSN 0132-344X. (In Russian). Chem. Abstr. 1982, 96, 173300.








| Na(hfac) | Ground Mixture, 3 h | Ground Mixture, 4 h | Pt(hfac)2 | Assignment |
|---|---|---|---|---|
| 1673 s | 1669 m | 1672 m | 1681 w | ν(C…O) |
| 1656 s | 1657 m | 1650 m | ν(C…C) | |
| 1555 w 1533 m 1514 sh 1490 m | 1553 w 1531 m 1513 w 1494 m | 1553 w 1531 m 1493 m | 1586 m 1560 vw 1535 vw 1436 m | ν(C…O) + ν(C…C…C) |
| 1334 w | 1332 w | 1320 w | 1348 w | νs(C–CF3) |
| 1259 m 1205 m wide | 1259 m 1191 m | 1256 m 1212 m | 1257 m 1200 m | ν(C–CF3), ν(C–F), ν(C…C…C) |
| 1131 vs | 1127 vs | 1135 vs | 1146 vs | δ(C–H) in plane |
| 1087 vw | 1087 vw | 1079 w | 1102 s | |
| 948 vw | 948 vw | 946 w | 955 vw | ν(C…O) |
| 847 vw 802 m | 852 w 794 m | 852 w 792 m | 833 vw 818 m | δ as(C–CF3) |
| 763 vw | 763 vw | 754 w | δ s(C–CF3) | |
| 739 m | 739 m | 739 m | 721 m | Ring def. |
| 663 m | 663 m | 663 m | ||
| 579 m | 579 m | 580 m | 612 m |
| Na(tfac) | Ground Mixture, 4 h | Pt(tfac)2 | Assignment |
|---|---|---|---|
| 1694 vw | 1686 s, 1668 s | ν(C…O) | |
| 1636 s | 1611 s | 1583 s | ν(C…O) |
| 1517 m 1495 s | 1525 s | 1523 m | ν(C…C…C) |
| 1429 w | 1449 m | 1450 m | δas(CH3) |
| 1363 w | 1363 m | 1367 m | δs(CH3) |
| 1287 s 1272 s | 1287 m | 1304 s | ν(C…C) + ν(C–CH3) |
| 1225 w, 1217 w, 1178 m, 1135 s | 1192 s 1137 s 1090 m | 1234 s, 1188 m, 1158 s, 1142 vs | ν(C–F) + ν(C–CF3) |
| 1020 w | 1024 w 1001 w | 1025 w | ρr(CH3) |
| 994 w | 949 m | 948 w | ν(C–CH3) + ν(C…O) |
| 845 m | 843 w | 882 m | |
| 765 m | 802 m | 801 m | δ as(C–CF3), δ(C–H) out-of-plane |
| 727 m | 725 m | 745 m 720 m | δ s(C–CF3) |
| 690 w | 662 w | 694 m | Ring def. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhaev, V.D.; Petrova, L.A. Mechanically Stimulated Solid-State Interaction of Platinum Tetrachloride with Sodium β-Diketonates. Molecules 2023, 28, 3496. https://doi.org/10.3390/molecules28083496
Makhaev VD, Petrova LA. Mechanically Stimulated Solid-State Interaction of Platinum Tetrachloride with Sodium β-Diketonates. Molecules. 2023; 28(8):3496. https://doi.org/10.3390/molecules28083496
Chicago/Turabian StyleMakhaev, Victor D., and Larisa A. Petrova. 2023. "Mechanically Stimulated Solid-State Interaction of Platinum Tetrachloride with Sodium β-Diketonates" Molecules 28, no. 8: 3496. https://doi.org/10.3390/molecules28083496
APA StyleMakhaev, V. D., & Petrova, L. A. (2023). Mechanically Stimulated Solid-State Interaction of Platinum Tetrachloride with Sodium β-Diketonates. Molecules, 28(8), 3496. https://doi.org/10.3390/molecules28083496