Novel Biologically Active N-Substituted Benzimidazole Derived Schiff Bases: Design, Synthesis, and Biological Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
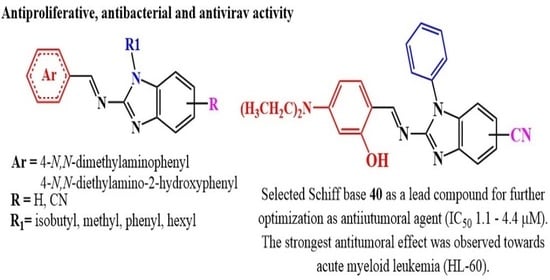
2.2. Biological Activity
2.2.1. Cytotoxicity and Antiviral Activity
2.2.2. Antibacterial Activity
2.2.3. Antiproliferative Activity
3. Conclusions
4. Materials and Methods
4.1. General Methods
4.2. Synthesis
4.2.1. General Method for Preparation of Compounds 7–8
N-Hexyl-2-nitroaniline 7
3-N-(Hexylamino)-4-nitrobenzonitrile 8
4.2.2. General Method for Preparation of Compounds 15–16
N1-Hexylbenzene-1,2-diamine 15
3-Amino-4-N-(hexylamino)benzonitrile 16
4.2.3. General Method for Preparation of Compounds 23 and 24
2-Amino-1-hexylbenzimidazole 23
2-Amino-6-cyano-1-hexylbenzimidazole 24
4.2.4. General Method for Preparation of Schiff Bases 28–45
(E)-4-(((1-Isobutyl-1H-benzo[d]imidazol-2-yl)imino)methyl)-N,N-dimethylaniline 28
(E)-2-((4-(Dimethylamino)benzylidene)amino)-1-isobutyl-1H-benzo[d]imidazole-6-carbonitrile 29
(E)-5-(Diethylamino)-2-(((1-isobutyl-1H-benzo[d]imidazol-2-yl)imino)methyl)phenol 30
(E)-2-((4-(Diethylamino)-2-hydroxybenzylidene)amino)-1-isobutyl-1H-benzo[d]imidazole-6-carbonitrile 31
(E)-1-Isobutyl-2-((4-nitrobenzylidene)amino)-1H-benzo[d]imidazole-6-carbonitrile 32
(E)-N,N-Dimethyl-4-(((1-methyl-1H-benzo[d]imidazol-2-yl)imino)methyl)aniline 33
(E)-2-((4-(Dimethylamino)benzylidene)amino)-1-methyl-1H-benzo[d]imidazole-6-carbonitrile 34
(E)-5-(Diethylamino)-2-(((1-methyl-1H-benzo[d]imidazol-2-yl)imino)methyl)phenol 35
(E)-2-((4-(Diethylamino)-2-hydroxybenzylidene)amino)-1-methyl-1H-benzo[d]imidazole-6-carbonitrile 36
(E)-1-Methyl-2-((4-nitrobenzylidene)amino)-1H-benzo[d]imidazole-6-carbonitrile 37
(E)-N,N-Dimethyl-4-(((1-phenyl-1H-benzo[d]imidazol-2-yl)imino)methyl)aniline 38
(E)-2-((4-(Dimethylamino)benzylidene)amino)-1-phenyl-1H-benzo[d]imidazole-6-carbonitrile 39
(E)-5-(Diethylamino)-2-(((1-phenyl-1H-benzo[d]imidazol-2-yl)imino)methyl)phenol 40
(E)-2-((4-(Diethylamino)-2-hydroxybenzylidene)amino)-1-phenyl-1H-benzo[d]imidazole-6-carbonitrile 41
(E)-4-(((1-Hexyl-1H-benzo[d]imidazol-2-yl)imino)methyl)-N,N-dimethylaniline 42
(E)-2-((4-(Dimethylamino)benzylidene)amino)-1-hexyl-1H-benzo[d]imidazole-6-carbonitrile 43
(E)-5-(Diethylamino)-2-(((1-hexyl-1H-benzo[d]imidazol-2-yl)imino)methyl)phenol 44
(E)-2-((4-(Diethylamino)-2-hydroxybenzylidene)amino)-1-hexyl-1H-benzo[d]imidazole-6-carbonitrile 45
4.3. Biology
4.3.1. Antiviral Activity
4.3.2. Antibacterial Activity
Materials
Methods
4.3.3. Cell Culture and Reference Compounds
4.3.4. Proliferation Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases-Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, T.T. Hugo (Ugo) Schiff, Schiff bases, and a century of β-lactam synthesis. Angew. Chem. Int. Ed. 2008, 47, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Arulmurugan, S.; Kavitha, H.P.; Venkatraman, B.R. Biological activities of Schiff base and its complexes: A review. Rasayan J. Chem. 2010, 3, 385–410. [Google Scholar]
- Qin, W.L.; Long, S.; Panunzio, M.; Biondi, S. Schiff bases: A short survey on anevergreen chemistry tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef]
- Zoubi, W.A. Biological Activities of Schiff Bases and Their Complexes: A Review of Recent Works. Int. J. Org. Chem. 2013, 3, 73–95. [Google Scholar] [CrossRef]
- Lenahan, C.; Sanghavi, R.; Huang, L.; Zhang, J.H. Rhodopsin: A Potential Biomarker for Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 326. [Google Scholar] [CrossRef]
- Carey, F.A. Organic Chemistry, 5th ed.; MacGraw-Hill: New York, NY, USA, 2003; p. 724. [Google Scholar]
- Anand, P.; Patil, V.M.; Sharma, V.K.; Khosa, R.L.; Masand, N. Schiff bases: A review on biological insights. Int. J. Drug Design Disc. 2012, 3, 851–865. [Google Scholar]
- Şener, N.; Özkinali, S.; Altunoglu, Y.C.; Yerlikaya, S.; Gökçe, H.; Zurnaci, M.; Gür, M.; Baloglu, M.C.; Şener, İ. Antiproliferative properties and structural analysis of newly synthesized Schiff bases bearing pyrazole derivatives and molecular docking studies. J. Mol. Struct. 2021, 1241, 130520. [Google Scholar] [CrossRef]
- Sztanke, K.; Maziarka, A.; Osinka, A.; Sztanke, M. An insight into synthetic Schiff bases revealing antiproliferative activities in vitro. Bioorg. Med. Chem. 2013, 21, 3648–3666. [Google Scholar] [CrossRef]
- Vicini, P.; Geronikaki, A.; Incerti, M.; Busonera, B.; Poni, G.; Cabras, C.A.; la Colla, P. Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg. Med. Chem. 2003, 11, 4785–4789. [Google Scholar] [CrossRef]
- Jos, S.; Suja, N.R. Chiral Schiff base ligands of salicylaldehyde: A versatile tool for medical applications and organic synthesis—A review. Inorg. Chim. Acta 2023, 547, 121323. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal-Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, T.; Ali, B.; Qayyum, H.; Haroone, M.S.; Shabbir, G. Pharmacological aspects of schiff base metal complexes: A critical review. Inorg. Chem. Comm. 2023, 150, 110449. [Google Scholar] [CrossRef]
- Saloutin, V.I.; Edilova, Y.O.; Kudyakova, Y.S.; Burgart, Y.V.; Bazhin, D.N. Heterometallic Molecular Architectures Based on Fluorinated β-Diketone Ligands. Molecules 2022, 27, 7894. [Google Scholar] [CrossRef] [PubMed]
- Cimerman, Z.; Snežana, M.; Nives, G. Schiff bases derived from aminopyridines as spectrofluorimetric analytical reagents. Croat. Chem. Acta 2000, 73, 85–96. [Google Scholar]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metalcomplexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Rauf, A.; Shah, A.; Khan, A.A.; Shah, A.H.; Abbasi, R.; Qureshi, I.Z.; Ali, S. Synthesis, pH dependent photometric and electrochemical investigation, redox mechanism and biological applications of novel Schiff base and its metallic derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 176, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Feng, C. Microwave-assisted synthesis, crystal structure and fluorescence of novel coordination complexes with Schiff base ligands. J. Mol. Struct. 2010, 977, 62–66. [Google Scholar] [CrossRef]
- Horak, E.; Kassal, P.; Hranjec, M.; Murković Steinberg, I. Benzimidazole functionalised Schiff bases: Novel pH sensitivefluorescence turn-on chromoionophores for ion-selective optodes. Sens. Act. B 2018, 258, 415–423. [Google Scholar] [CrossRef]
- Castillo-Martnez, E.; Carretero-Gonzlez, J.; Armand, M. Polymeric Schiff Bases as Low-Voltage Redox Centers for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2014, 53, 5341–5345. [Google Scholar] [CrossRef]
- Abdel Hameed, R.S. Schiff’ Bases as Corrosion Inhibitor for Aluminum Alloy in Hydrochloric Acid Medium. Tenside Surf. Det. 2019, 56, 3–10. [Google Scholar] [CrossRef]
- Gama, S.; Mendes, F.; Marques, F.; Santos, I.C.; Carvalho, M.F.; Correia, I.; Pessoa, J.C.; Santos, I.; Paulo, A. Copper(II) complexes with tridentate pyrazole-based ligands: Synthesis, characterization, DNA cleavage activity and cytotoxicity. J. Inorg. Biochem. 2011, 105, 637–644. [Google Scholar] [CrossRef]
- Katwal, R.; Kaur, H.; Kapur, B.K. Applications of copper—Schiff’s base complexes: A review. Sci. Rev. Chem. Commun. 2013, 3, 1–15. [Google Scholar]
- Aiyelabola, T.; Jordaan, J.; Otto, D.; Akinkunmi, E. Syntheses, Characterization, Antimicrobial Activity and Extraction Studies of Tetraaza Macrocyclic/Linear Schiff Bases Derived from Benzene-1,4-Dicarboxaldehyde and Their Coordination Compounds. Adv. Biolog. Chem. 2021, 11, 79–105. [Google Scholar] [CrossRef]
- Fonkui, T.Y.; Ikhile, M.I.; Ndinteh, D.T.; Njobeh, P.B. Microbial activity of some heterocyclic Schiff bases and metal complexes: A review. Trop. J. Pharm. Res. 2018, 17, 2507–2518. [Google Scholar] [CrossRef]
- Ugras, H.I.; Basaran, I.; Kilic, T.; Cakir, U. Synthesis, Complexation and Antifungal, Antibacterial Activity Studies of a New Macrocyclic Schiff Base. J. Het. Chem. 2006, 43, 1679–1684. [Google Scholar] [CrossRef]
- Singh, H.L. Synthesis and characterization of tin (II) complexes of fluorinated Schiff bases derived from amino acids. Spect. Acta Part A 2010, 76, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Bhattacharjee, A.; Roy, A.; Chatterjee, S.; Roy, P. Chromogenic andfluorescence sensing of pH with a Schiff-base molecule. RSC Adv. 2016, 6, 39118–39124. [Google Scholar] [CrossRef]
- Hameed Haddad, H. A New Schiff Base Derivatives Designed to Bind Metal Ion (Cu, Co): Thermodynamics and Biological Activity Studies. J. Anal. Chem. 2016, 7, 445–451. [Google Scholar] [CrossRef]
- Nawrocka, W.; Sztuba, B.; Kowalska, M.W.; Liszkiewicz, H.; Wietrzyk, J.; Nasulewicz, A.; Pełczynska, M.; Opolski, A. Synthesis and Antiproliferative Activity in vitro of New 2-Aminobenzimidazole Derivatives Part 2 [1]. Farmaco 2004, 59, 1047–1055. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zarganes-Tzitzikas, T.; Tsoleridis, C.A.; Stephanidou-Stephanatou, J.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J.; Choli-Papadopoulou, T. One-pot microwave assisted synthesis under green chemistry conditions, antioxidant screening, and cytotoxicity assessments of benzimidazole Schiff bases and pyrimido[1,2-a]benzimidazol-3(4H)-ones. Eur. J. Med. Chem. 2011, 46, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Fonkui, T.Y.; Ikhile, M.I.; Njobeh, P.B.; Ndinteh, D.T. Benzimidazole Schiff base derivatives: Synthesis, characterization and antimicrobial activity. BMC Chem. 2019, 13, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.A.M.F.; Ahmad, T.; Nazmuzzaman, M.; Ray, S.K.; Sharifuzzaman, M.; Karim, M.R.; Alam, M.G.; Ajam, M.M.; Maitra, P.; Mandol, D.; et al. Synthesis of Benzimidazole Derivatives Containing Schiff Base Exhibiting Antimicrobial Activities. Int. J. Res. Stud. Biosci. 2017, 5, 18–24. [Google Scholar]
- Kumaravel, G.; Utthra, P.P.; Raman, N. Exploiting the biological efficacy of benzimidazole based Schiff base complexes with L-Histidine as a co-ligand: Combined molecular docking, DNA interaction, antimicrobial and cytotoxic studies. Bioorg. Chem. 2018, 77, 269–279. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Liscano, Y.; Upegui, Y.; Robledo, S.M.; Ramírez-Apan, M.T.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. In Vitro Evaluation of the Potential Pharmacological Activity and Molecular Targets of New Benzimidazole-Based Schiff Base Metal Complexes. Antibiotics 2021, 10, 728. [Google Scholar] [CrossRef]
- Song, W.-J.; Cheng, J.-P.; Jiang, D.-H.; Guo, L.; Cai, M.-F.; Yang, H.-B.; Lin, Q.-Y. Synthesis, interaction with DNA and antiproliferative activities of twonovel Cu(II) complexes with Schiff base of benzimidazole. Spect. Acta Part A Mol. Biomol. Spectr. 2014, 121, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hranjec, M.; Starčević, K.; Kraljević Pavelić, S.; Lučin, P.; Pavelić, K.; Karminski Zamola, G. Synthesis, spectroscopic characterization and antiproliferative evaluation in vitro of novel Schiff bases related to benzimidazoles. Eur. J. Med. Chem. 2011, 46, 2274–2279. [Google Scholar] [CrossRef]
- Grgičević, I.; Mikulandra, I.; Bukvić, M.; Banjanac, M.; Radovanović, V.; Habinovec, I.; Bertoša, B.; Novak, P. Discovery of macrozones, new antimicrobial thiosemicarbazone-based azithromycin conjugates: Design, synthesis and in vitro biological evaluation. Int. J. Antimicrob. Agents 2020, 56, 106147. [Google Scholar] [CrossRef]




| Cpd | Cytotoxicity (CC50/µM) | Antiviral Activity (EC50/μM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HEL 229 | Huh-7 | MDCK | HCoV 229E HEL 299 | HCoV OC43 HEL 299 | HCoV NL63 Huh-7 | Influenza H1N1 MDCK | Influenza H3N2 MDCK | Influenza B MDCK | RSV A Long HEL 299 | HSV-1 KOS HEL 299 | YFV 17D Huh-7 | Zika Mr776 Huh-7 | Sindbis Huh-7 | |
| 17 | >100 | >100 | >100 | 78.9 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 18 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 43.1 | >100 |
| 22 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 78.7 | >100 | >100 | 46.4 | >100 |
| 29 | >100 | >100 | >100 | >100 | >100 | 79.8 | 94.1 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 31 | >100 | 85.7 | 1.3 | >100 | >100 | 32 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 32 | >100 | 90.1 | >100 | 38.5 | 68.7 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 34 | >100 | >100 | >100 | >100 | >100 | 82.8 | >100 | >100 | 83.5 | >100 | >100 | >100 | >100 | >100 |
| 36 | >100 | >100 | >100 | 88.4 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 38 | >100 | >100 | >100 | >100 | 94.8 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 41 | >100 | >100 | 71.6 | >100 | 89.5 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 42 | >100 | 2.7 | 34.6 | 34.7 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| Remdesivir | >10 | >10 | - | 0.06 | 0.06 | 0.03 | - | - | - | 0.03 | - | 6.2 | 0.7 | >10 |
| Ribavirin | >250 | 8.9 | 67.0 | 82.6 | 170.1 | >250 | 10.5 | 4.0 | 2.8 | 10.8 | - | >250 | >250 | 148.1 |
| Zanamivir | - | - | >100 | - | - | - | 0.1 | 16.8 | 0.05 | - | - | - | - | - |
| Rimantadine | - | - | >100 | - | - | - | 5.0 | 0.05 | >100 | - | - | - | - | - |
| BVDU | >100 | - | - | - | - | - | - | - | - | - | 0.05 | - | - | - |
| Cpd | S. aureus ATCC 29213 | E. faecalis ATCC 29212 | S. pneumoniae ATCC 49619 | E. coli ATCC 25922 | E. coli efflux del | P. aeruginosa ATCC 27853 | K. pneumoniae ATCC 700603 | A. baumannii ATCC 17978 |
|---|---|---|---|---|---|---|---|---|
| 23 | 16 | 32 | 32 | 64 | 32 | >64 | >64 | 64 |
| 37 | 32 | 64 | 64 | >64 | >64 | >64 | >64 | >64 |
| 38 | >64 | 32 | >64 | >64 | >64 | >64 | >64 | >64 |
| 41 | >64 | 32 | 64 | >64 | >64 | >64 | >64 | >64 |
| 42 | 32 | 32 | 64 | >64 | 64 | >64 | >64 | >64 |
| Ampicillin | 0.5 | 2 | 2 | <0.125 | 0.5 | >64 | >64 | >64 |
| Ceftazidime | 8 | <0.125 | 0.25 | 0.5 | >64 | 1 | 64 | 32 |
| Ciprofloxacin | 0.125 | <0.125 | <0.125 | 0.5 | 1 | 0.25 | 0.25 | 4 |
| Meropenem | <0.125 | <0.125 | <0.125 | <0.125 | 8 | 1 | 0.25 | <0.125 |
| Cpd | IC50 (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| LN-229 | Capan-1 | HCT-116 | NCI-H460 | DND-41 | HL-60 | K-562 | Z-138 | |
| 19 | >100 | >100 | >100 | >100 | 98.3 | >100 | >100 | >100 |
| 21 | >100 | >100 | >100 | 69.2 | >100 | >100 | >100 | >100 |
| 23 | 45.2 | 46.5 | 57.4 | 60.2 | 47.7 | 44.9 | 46.7 | 44.7 |
| 28 | >100 | >100 | 15.2 | >100 | >100 | >100 | >100 | >100 |
| 29 | >100 | >100 | >100 | >100 | >100 | 42.8 | >100 | >100 |
| 30 | >100 | >100 | >100 | >100 | >100 | 70.4 | >100 | >100 |
| 31 | >100 | 32.8 | >100 | >100 | 34.4 | 47.6 | >100 | >100 |
| 32 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 33 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 34 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 35 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 36 | >100 | >100 | >100 | >100 | 41.4 | >100 | >100 | >100 |
| 37 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 38 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 39 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 40 | 21.5 | 2.4 | 9.6 | 43.7 | 2.2 | 1.1 | 12.9 | 4.4 |
| 41 | >100 | 69.5 | >100 | >100 | 76.3 | 49.6 | >100 | 35.2 |
| 42 | >100 | >100 | >100 | 64.4 | >100 | 73.2 | 90.8 | >100 |
| 43 | >100 | >100 | >100 | 91.6 | >100 | 33.5 | >100 | >100 |
| 44 | >100 | >100 | >100 | >100 | >100 | 83.9 | >100 | >100 |
| 45 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| ETP | 2.40 | 0.43 | 1.45 | 3.65 | 2.80 | 0.42 | 1.77 | 0.85 |
| NZO | 0.29 | 0.10 | 0.13 | 0.25 | 0.47 | 0.10 | 0.07 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beč, A.; Cindrić, M.; Persoons, L.; Banjanac, M.; Radovanović, V.; Daelemans, D.; Hranjec, M. Novel Biologically Active N-Substituted Benzimidazole Derived Schiff Bases: Design, Synthesis, and Biological Evaluation. Molecules 2023, 28, 3720. https://doi.org/10.3390/molecules28093720
Beč A, Cindrić M, Persoons L, Banjanac M, Radovanović V, Daelemans D, Hranjec M. Novel Biologically Active N-Substituted Benzimidazole Derived Schiff Bases: Design, Synthesis, and Biological Evaluation. Molecules. 2023; 28(9):3720. https://doi.org/10.3390/molecules28093720
Chicago/Turabian StyleBeč, Anja, Maja Cindrić, Leentje Persoons, Mihailo Banjanac, Vedrana Radovanović, Dirk Daelemans, and Marijana Hranjec. 2023. "Novel Biologically Active N-Substituted Benzimidazole Derived Schiff Bases: Design, Synthesis, and Biological Evaluation" Molecules 28, no. 9: 3720. https://doi.org/10.3390/molecules28093720
APA StyleBeč, A., Cindrić, M., Persoons, L., Banjanac, M., Radovanović, V., Daelemans, D., & Hranjec, M. (2023). Novel Biologically Active N-Substituted Benzimidazole Derived Schiff Bases: Design, Synthesis, and Biological Evaluation. Molecules, 28(9), 3720. https://doi.org/10.3390/molecules28093720