A Remarkable Difference in Pharmacokinetics of Fluorinated Versus Iodinated Photosensitizers Derived from Chlorophyll-a and a Direct Correlation between the Tumor Uptake and Anti-Cancer Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photophysical Properties of Photosensitizers
2.2. Biological Studies
2.3. Intracellular Localization of PSs in UMUC3 (Bladder) and U87 (Brain) Tumor Cells
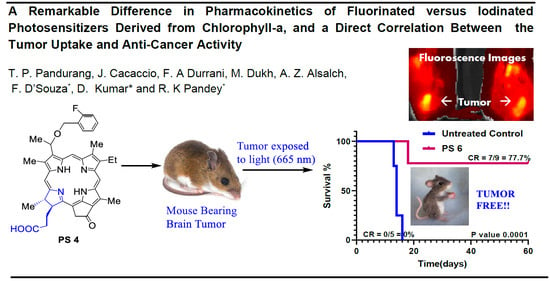
2.4. In Vivo Uptake and PDT Efficacy
2.5. Comparative In Vivo PDT Efficacy
3. Conclusions
4. Experimental Section
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Ahmed, F.B.; Anderson, R.N. The leading causes of death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Dotto, G.P. Racial differences in cancer susceptibility and survival: More than the color of the skin? Trends Cancer 2017, 3, 181–197. [Google Scholar] [CrossRef]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Calcaianu, N.; Popescu, S.A.; Diveica, D.; Lascar, L. Surgical attitude in premalignant lesions and malignant tumors of the lower lip. J. Med. Life 2015, 8, 109–111. [Google Scholar] [PubMed]
- Irani, S. Pre-cancerous lesions in the oral and maxillofacial region: A literature review with special focus on etiopathogenesis. Iran J. Pathol. 2016, 11, 303–322. [Google Scholar] [PubMed]
- Abola, M.V.; Prasad, V.; Jena, A.B. Association between treatment toxicity and outcomes on oncology clinical trials. Ann. Oncol. 2014, 25, 2284–2289. [Google Scholar] [CrossRef] [PubMed]
- Yoshi, Y.; Tashima, H.; Iwao, Y.; Yoshida, E.; Wakizaka, H.; Akamatsu, G.; Yamaya, T.; Matsumoto, H.; Yoshimoto, M.; Igarashi, C.; et al. Immuno-open PET: A novel approach for early diagnosis and image-guided surgery for small resectable pancreatic cancer. Nat. Sci. Rep. 2020, 10, 4143. [Google Scholar] [CrossRef]
- Kasban, H.; El-Bendary, M.A.M.; Salama, D.H. A comparative study of medical imaging techniques. Int. J. Inf. Sci. Intell. Syst. 2015, 4, 37–58. [Google Scholar]
- Ni, D.; Ehlerding, E.B.; Cai, W. Multimodality imaging agents with PET as the fundamental pillar. Angew. Chem. Int. Ed. Engl. 2019, 58, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shu, J. Multimodal molecular imaging: Current status and future directions. Contrast Media Mol. Imaging 2018, 2018, 1382183. [Google Scholar] [CrossRef] [PubMed]
- An, F.-F.; Chan, M.; Kommidi, H.; Ting, R. Dual PET and near-infrared fluorescence imaging probes as tools for imaging oncology. Am. J. Roentgenol. 2016, 207, 206–273. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Hicks, R.J. How we read oncologic FDG PET/CT. Cancer Imaging 2016, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Kawada, K.; Iwamoto, M.; Sakai, Y. Mechanisms underlying 18F-fluorodeoxyglucose in colorectal cancer. World J. Radiol. 2016, 28, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.; Igerc, I.; Beyer, T.; Reinprecht, P.; Hausegger, K. Advantages and limitations of PDG PET in the follow up of breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 5125–5134. [Google Scholar]
- Bouvhelouche, K.; Turkbey, B.; Choyke, P.L. PET/CT and MRI in bladder cancer. J. Cancer Sci. Ther. 2012, 14, 7692. [Google Scholar]
- Cascini, G.L.; Asabella, A.N.; Notaristefano, A.; Restuccia, A.; Ferrari, C.; Rubini, D.; Altni, C.; Rubini, G. 124Iodine: A longer-life positron emitter isotope—New Opportunities in Molecular Imaging. BioMed Res. Int. 2014, 2014, 672094. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor-imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Pan, D.; Liang, P.; Zhong, X.; Wang, D.; Cao, H.; Wang, W.; He, W.; Yang, Z.; Dong, X. Self-assembled porphyrin-based nanoparticles with enhanced near-infrared absorbance for fluorescence imaging and cancer photodynamic therapy. ACS Appl. Bio Mater. 2019, 2, 999–1005. [Google Scholar] [CrossRef]
- Yang, V.X.; Muller, P.J.; Herman, P.; Wilson, B.C. A multispectral fluorescence imaging system: Design and initial clinical tests in intra-operative Photofrin-photodynamic therapy of brain tumors. Lasers Surg. Med. 2003, 32, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Blanco, K.C.; Moriyama, L.T.; Inada, N.M.; Salnio, A.G.; Menezes, P.F.C.; Leite, E.J.S.; Kurachi, C.; Bagnato, V.S. Fluorescence guided PDT for optimization of the outcome of skin cancer treatment. Front. Phys. 2015, 3, 30. [Google Scholar] [CrossRef]
- Ma, B.; Li, G.; Kanter, P.; Lamonica, D.; Grossman, Z.; Pandey, R.K. Bifunctional HPPH-N2S2 Tc conjugates as tumor imaging agents: Synthesis and biodistribution studies. J. Porphyr. Phthalocyanines 2003, 7, 500–507. [Google Scholar] [CrossRef]
- Pandey, S.K.; Gryshuk, A.L.; Sajjad, M.; Zheng, X.; Chen, Y.; Abouzeid, M.H.; Morgan, J.; Charamisinau, I.; Nabi, H.A.; Oseroff, A.; et al. Multimodality agents for tumor imaging (PET, Fluorescence) and photodynamic therapy. A possible “See and Treat” approach. J. Med. Chem. 2005, 48, 6286–6295. [Google Scholar] [CrossRef] [PubMed]
- Marko, A.J.; Borah, B.M.; Siters, K.E.; Missert, J.R.; Gupta, A.; Pera, P.; Isaac-Lam, M.E.; Pandey, R.K. Targeted Nanoparticles for fluorescence imaging of folate receptor positive tumors. Biomolecules 2020, 10, 1651. [Google Scholar] [CrossRef]
- Zheng, S.; Cheruku, R.R.; Dukh, M.; Tabaczynski, W.; Patel, N.J.; White, W.H.; Missert, J.R.; Spernyak, J.A.; Pandey, R.K. The structure of Gd(III) chelates conjugated at the periphery of HPPH have a significant impact on the imaging and therapy of cancer. ChemMedChem 2020, 15, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Wang, S.; Marko, A.; Joshi, P.; Ethirajan, M.; Chen, Y.; Yao, R.; Sajjad, M.; Kopelman, R.; Pandey, R.K. Polyacrylamide-based biocompitable nanoplatform enhances the tumor uptake, PET/fluorescence imaging and anticancer activity of a chlorophyll analog. Theranostics 2014, 16, 614–628. [Google Scholar] [CrossRef] [PubMed]
- James, N.S.; Chen, Y.; Ohulchanskyy, T.Y.; Ethirajan, M.; Henary, M.; Strekowsk, L.; Pandey, R.K. Evaluation of polymethine dyes as potential probes for new infrared fluorescence imaging of tumor: Part-1. Theranostics 2013, 3, 692–702. [Google Scholar] [CrossRef]
- Kim, S.; Yang, J.; Ahn, J.-H.; Ko, I.O.; Kim, J.Y.; Lee, Y.J.; Park, J.A. Porphyrin-based tumor-targeting theranostic agent: Gd-TDAP. ACS Med. Chem. Lett. 2021, 12, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Srivatsan, A.; Pera, P.; Joshi, P.; Marko, A.J.; Durrani, F.; Missert, J.R.; Curtin, L.; Sexton, S.; Yao, R.; Sajjad, M.; et al. Highlights on the imaging (nuclear and fluorescence) and phototherapeutic potential of a tri-functional chlorophyll-a analog with no significant toxicity in mice and rats. J. Photochem. Photobiol. B Biol. 2020, 211, 111988. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, A.; Khan, S.A.; Bjarwani, N.; Stewart, V.; Rockil, A.G.; Khan, S.; Barwick, T.D. FDG PET/CT pitfalls in gynecologic and genitourinary oncologic imaging. RadioGraphics 2017, 37, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Suomi, Y.L.; Shibata, N. Contribution of organofluorine compounds in pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Johnson, B.M.; Shu, Y.-Z.; Zhou, X.; Meanwell, N.A. Metabolic and pharmaceutical aspects of fluorinated compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef] [PubMed]
- Cheruku, R.R.; Tracy, E.C.; Tabaczynski, W.; Missert, J.R.; Baumann, H.; Pandey, R.K. Chiral alkyl groups at position 3(1’) of Pyropheophorbide-a specify uptake and retention by tumor cells and are essential for effective photodynamic therapy. J. Med. Chem. 2021, 64, 4787–4809. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Singapore, 2005. [Google Scholar]
- Blazquez-Castro, A. Direct 1O2 optical excitation: A tool for redox biology. Redox. Biol. 2017, 13, 39–59. [Google Scholar] [PubMed]
- Clo, E.; Snyder, J.W.; Ogilby, R.; Gothelf, K.V. Control and selectivity of photosensitized singlet oxygen production: Challenges in complex biological systems. ChemMedChem 2007, 8, 475–481. [Google Scholar] [CrossRef]
- Dukh, M.; Tabaczynski, W.A.; Seetharaman, S.; Ou, Z.; Kadish, K.M.; D’Souza, F.; Pandey, R.K. Meso- and β-pyrrole-linked chlorin-bacteriochlorin dyads for promoting far-red FRET and singlet oxygen production. Chem. Eur. J. 2020, 26, 14996–15006. [Google Scholar] [CrossRef]
- Seetharaman, S.; Dukh, M.; Tabaczynski, W.A.; Ou, Z.; Karr, P.A.; Kadish, K.M.; Pandey, R.K.; D’Souza, F. Meso-biphenyl-linked NIR- and far-infrared emitting, chlorin and bacteriochlorin dimers: Synthesis, excitation transfer, and singlet oxygen production. ChemPlusChem 2021, 86, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Correlation between subcellular localization and photodynamic therapy. J. Porphyr. Phthalocyanines 2004, 8, 1009–1014. [Google Scholar] [CrossRef]
- Kessel, D.; Vicente, M.G.; Reiners, J.J., Jr. Initiation of apoptosis and autophagy by photodynamic therapy. Autophagy 2006, 2, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Cavaleiro, J.A.S.; Gorner, H.; Lacerda, P.S.S.; MacDonald, J.G.; Mark, G.; Neves, M.G.P.M.S.; Nohr, R.S.; Schuchmann, H.-P.; von Sonntag, C.; Tome, A.C. Singlet oxygen formation and photostability of meso-tetraarylporphyrin derivatives and their copper complexes. J. Photochem. Photobiol. A Chem. 2001, 144, 131–140. [Google Scholar] [CrossRef]
- Pineiro, M.; Carvalho, A.L.; Pereira, M.M.; Gonsalves, A.M.d.A.R.; Arnaut, L.G.; Formosinho, S. Photoacoustic measurements of porphyrin triplet-state quantum yields and singlet oxygen efficiencies. Chem. Eur. J. 1998, 4, 2299–2307. [Google Scholar] [CrossRef]









| Compound | λabs, nm | λem, nm | τav, ns | QYF | QYSO |
|---|---|---|---|---|---|
| 2 | 410, 504, 537, 606, 664 | 665, 697 | 6.30 | 0.043 | 0.488 |
| 3 | 411, 504, 534, 605, 663 | 664, 697 | 6.89 | 0.043 | 0.466 |
| 4 | 411, 504, 534, 605, 663 | 664, 697 | 8.33 | 0.050 | 0.500 |
| 5 | 410, 503, 534, 606, 664 | 664, 697 | 6.92 | 0.041 | 0.489 |
| 6 | 410, 503, 536, 606, 664. | 664, 697 | 6.72 | 0.038 | 0.430 |
| 7 | 410, 503, 535, 604, 664 | 664, 697 | 6.68 | 0.046 | 0.437 |
| 8 | 411, 503, 536, 604, 643 | 664, 697 | 6.68 | 0.046 | 0.487 |
| PS | IC50 (mM) UMUC3 | IC50 (mM) U87 | PS | IC50 (mM) UMUC3 | IC50 (mM) U87 |
|---|---|---|---|---|---|
| 2 | 0.095 | 0.534 | 6 | 0.009 | 0.202 |
| 3 | 0.022 | 0.227 | 7 | 0.048 | 0.303 |
| 4 | 0.014 | 0.145 | 8 | 0.027 | 0.027 |
| 5 | 0.018 | 0.284 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandurang, T.P.; Cacaccio, J.; Durrani, F.A.; Dukh, M.; Alsaleh, A.Z.; Sajjad, M.; D’Souza, F.; Kumar, D.; Pandey, R.K. A Remarkable Difference in Pharmacokinetics of Fluorinated Versus Iodinated Photosensitizers Derived from Chlorophyll-a and a Direct Correlation between the Tumor Uptake and Anti-Cancer Activity. Molecules 2023, 28, 3782. https://doi.org/10.3390/molecules28093782
Pandurang TP, Cacaccio J, Durrani FA, Dukh M, Alsaleh AZ, Sajjad M, D’Souza F, Kumar D, Pandey RK. A Remarkable Difference in Pharmacokinetics of Fluorinated Versus Iodinated Photosensitizers Derived from Chlorophyll-a and a Direct Correlation between the Tumor Uptake and Anti-Cancer Activity. Molecules. 2023; 28(9):3782. https://doi.org/10.3390/molecules28093782
Chicago/Turabian StylePandurang, Taur Prakash, Joseph Cacaccio, Farukh A. Durrani, Mykhaylo Dukh, Ajyal Z. Alsaleh, Munawwar Sajjad, Francis D’Souza, Dalip Kumar, and Ravindra K. Pandey. 2023. "A Remarkable Difference in Pharmacokinetics of Fluorinated Versus Iodinated Photosensitizers Derived from Chlorophyll-a and a Direct Correlation between the Tumor Uptake and Anti-Cancer Activity" Molecules 28, no. 9: 3782. https://doi.org/10.3390/molecules28093782
APA StylePandurang, T. P., Cacaccio, J., Durrani, F. A., Dukh, M., Alsaleh, A. Z., Sajjad, M., D’Souza, F., Kumar, D., & Pandey, R. K. (2023). A Remarkable Difference in Pharmacokinetics of Fluorinated Versus Iodinated Photosensitizers Derived from Chlorophyll-a and a Direct Correlation between the Tumor Uptake and Anti-Cancer Activity. Molecules, 28(9), 3782. https://doi.org/10.3390/molecules28093782