Electrochemical Sensor for Tryptophan Determination Based on Trimetallic-CuZnCo-Nanoparticle-Modified Electrodes
Abstract
:1. Introduction
2. Results
2.1. Electrochemical, Morphological, and Structural Characterization of Deposited Metallic Nanoparticles
2.2. Electrocatalytic Measurement of Tryptophan
2.3. Influence of Scan Rate on the Voltametric Response of Tryptophan
2.4. Analytical Performances of the CuZnCo-Modified Electrode toward Tryptophan
2.5. Electrode Stability and Repeatability
2.6. Effect of pH Values
2.7. Interference Studies
2.8. Real Sample Analysis
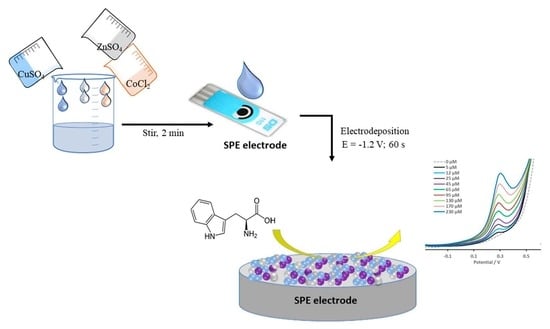
3. Materials and Methods
3.1. Reagents
3.2. Apparatuses and Methods
3.3. Modification of the Electrode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emery, P.W. Amino acids: Metabolism. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 72–78. [Google Scholar]
- Bell, C.; Abrams, J.; Nutt, D. Tryptophan depletion and its implications for psychiatry. Br. J. Psychiatry 2001, 178, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Analysis, nutrition, and health benefits of tryptophan. Int. J. Tryptophan Res. 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sadok, I.; Gamian, A.; Staniszewska, M.M. Chromatographic analysis of tryptophan metabolites. J. Sep. Sci. 2017, 40, 3020–3045. [Google Scholar] [CrossRef] [PubMed]
- Karakawa, S.; Nishimoto, R.; Harada, M.; Arashida, N.; Nakayama, A. Simultaneous analysis of tryptophan and its metabolites in human plasma using liquid chromatography–electrospray ionization tandem mass spectrometry. Chromatography 2019, 40, 127–133. [Google Scholar] [CrossRef]
- Laich, A.; Neurauter, G.; Widner, B.; Fuchs, D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin. Chem. 2002, 48, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Vignau, J.; Jacquemont, M.C.; Lefort, A.; Imbenotte, M.; Lhermitte, M. Simultaneous determination of tryptophan and kynurenine in serum by HPLC with UV and fluorescence detection. Biomed. Chromatogr. 2004, 18, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, W.G.; Jyoti, A.; Mishra, M.K.; Louissaint, N.; Romero, R.; Chugani, D.C.; Kannan, S.; Kannan, R.M. Concurrent quantification of tryptophan and its major metabolites. Anal. Biochem. 2013, 443, 222–231. [Google Scholar] [CrossRef]
- Vaarmann, A.; Kask, A.; Mäeorg, U. Novel and sensitive high-performance liquid chromatographic method based on electrochemical coulometric array detection for simultaneous determination of catecholamines, kynurenine and indole derivatives of tryptophan. J. Chromatogr. B 2002, 769, 145–153. [Google Scholar] [CrossRef]
- Du, T.-T.; Cui, T.; Qiu, H.-M.; Wang, N.-R.; Huang, D.; Jiang, X.-H. Simultaneous determination of tryptophan, kynurenine, kynurenic acid and two monoamines in rat plasma by HPLC-ECD/DAD. J. Pharm. Biomed. Anal. 2018, 158, 8–14. [Google Scholar] [CrossRef]
- Bech-Andersen, S. Determination of Tryptophan with HPLC after Alkaline Hydrolysis in Autoclave using α-methyl-tryptophan as Internal Standard. Acta Agric. Scand. 1991, 41, 305–309. [Google Scholar] [CrossRef]
- Brabec, V.; Mornstein, V. Electrochemical behavior of proteins at graphite electrodes: II. Electrooxidation of aminoacids. Biophys. Chem. 1980, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Malfoy, B.; Reynaud, J.A. Electrochemical investigations of amino acids at solid electrodes: II amino acids containing no sulfur atoms: Tryptophan, tyrosine, histidine and derivatives. J. Electroanal. Chem. 1980, 114, 213. [Google Scholar] [CrossRef]
- Nasimi, H.; Madsen, J.S.; Zedan, A.H.; Malmendal, A.; Osther, P.J.S.; AlZahra’a Alatraktchi, F. Electrochemical sensors for screening of tyrosine and tryptophan as biomarkers for diseases: A narrative review. Microchem. J. 2023, 190, 108737. [Google Scholar] [CrossRef]
- Mao, K.; Yang, Z.; Li, J.; Zhou, X.; Li, X.; Hu, J. A novel colorimetric biosensor based on non-aggregated Au@ Ag core–shell nanoparticles for methamphetamine and cocaine detection. Talanta 2017, 175, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.V.; Karthik, R.; Chen, S.-M.; Marikkani, S.; Elangovan, A.; Muthuraj, V. Green synthesis of a novel flower-like cerium vanadate microstructure for electrochemical detection of tryptophan in food and biological samples. J. Colloid Interface Sci. 2017, 496, 78–86. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Zhang, X. Electrode material fabricated by loading cerium oxide nanoparticles on reduced graphene oxide and its application in electrochemical sensor for tryptophan. J. Alloys Compd. 2020, 842, 155934. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L. Electrochemical sensor for tryptophan determination based on copper-cobalt hexacyanoferrate film modified graphite electrode. Sensors 2007, 7, 2446–2457. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.-H.; Lu, H.-T.; Zhang, Q. Electrochemistry and voltammetric determination of L-tryptophan and L-tyrosine using a glassy carbon electrode modified with a Nafion/TiO2-graphene composite film. Microchim. Acta 2011, 173, 241–247. [Google Scholar] [CrossRef]
- Gao, J.; Li, H.; Li, M.; Wang, G.; Long, Y.; Li, P.; Li, C.; Yang, B. Polydopamine/graphene/MnO2 composite-based electrochemical sensor for in situ determination of free tryptophan in plants. Anal. Chim. Acta 2021, 1145, 103–113. [Google Scholar] [CrossRef]
- Sundaresan, R.; Mariyappan, V.; Chen, S.-M.; Keerthi, M.; Ramachandran, R. Electrochemical sensor for detection of tryptophan in the milk sample based on MnWO4 nanoplates encapsulated RGO nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126889. [Google Scholar] [CrossRef]
- He, Q.; Tian, Y.; Wu, Y. Electrochemical Sensor for Rapid and Sensitive Detection of Tryptophan by a Cu2O Nanoparticles-Coated Reduced Graphene Oxide Nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, J.; Xu, Z.; Liu, M.; Tang, S.; Yang, C.; Qian, D. Facile synthesis of Pd−Cu@Cu2O/N-RGO hybrid and its application for electrochemical detection of tryptophan. Electrochim. Acta 2018, 260, 526–535. [Google Scholar] [CrossRef]
- Arvinte, A.; Sesay, A.-M.; Virtanen, V. Carbohydrates electrocatalytic oxidation using CNT–NiCo-oxide modified electrodes. Talanta. 2011, 84, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Maduraiveeran, G. Bimetallic Nanomaterials-Based Electrochemical Biosensor Platforms for Clinical Applications. Micromachines 2021, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Luque, R.; Xu, G. Recent Advances in the Synthesis and Electrocatalytic Applications of Platinum-Based Bimetallic Alloy Nanostructures. Chem. Cat Chem. 2015, 7, 3206–3228. [Google Scholar] [CrossRef]
- Yeo, I.-H.; Johnson, D.C. Electrochemical response of small organic molecules at nickel–copper alloy electrodes. J. Electroanal. Chem. 2001, 495, 110–119. [Google Scholar] [CrossRef]
- Sun, Y.; Buck, H.; Mallouk, T.E. Combinatorial Discovery of Alloy Electrocatalysts for Amperometric Glucose Sensors. Anal. Chem. 2001, 73, 1599–1604. [Google Scholar] [CrossRef]
- Wang, J.; Thomas, D.F.; Chen, A. Nonenzymatic Electrochemical Glucose Sensor Based on Nanoporous PtPb Networks. Anal. Chem. 2008, 80, 997–1004. [Google Scholar] [CrossRef]
- Arvinte, A.; Crudu, I.-A.; Doroftei, F.; Timpu, D.; Pinteala, M. Electrochemical codeposition of silver-gold nanoparticles on CNT-based electrode and their performance in electrocatalysis of dopamine. J. Electroanal. Chem. 2018, 829, 184–193. [Google Scholar] [CrossRef]
- Arvinte, A.; Doroftei, F.; Pinteala, M. Comparative electrodeposition of Ni–Co nanoparticles on carbon materials and their efficiency in electrochemical oxidation of glucose. J. Appl. Electrochem. 2016, 46, 425–439. [Google Scholar] [CrossRef]
- Cui, H.-F.; Ye, J.-S.; Liu, X.; Zhang, W.-D.; Sheu, F.-S. Pt–Pb alloy nanoparticle/carbon nanotube nanocomposite: A strong electrocatalyst for glucose oxidation. Nanotechnology 2006, 17, 2334–2339. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Huang, S.; Li, M.; Guo, T.; Mo, C.; Pang, X.; Chen, L.; Li, X. In-situ facile preparation of highly efficient copper/nickel bimetallic nanocatalyst on chemically grafted carbon nanotubes for nonenzymatic sensing of glucose. J. Colloid Interface Sci. 2019, 557, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Zhang, Z.; Zhang, P.; Xiao, S.; Wang, L.; Dong, Y.; Yuan, H.; Li, P.; Sun, Y.; Jiang, X. 3D nanoporous gold scaffold supported on graphene paper: Freestanding and flexible electrode with high loading of ultrafine PtCo alloy nanoparticles for electrochemical glucose sensing. Anal. Chim. Acta 2016, 938, 63–71. [Google Scholar] [CrossRef]
- Xu, C.; Vasileff, A.; Jin, B.; Wang, D.; Xu, H.; Zheng, Y.; Qiao, S.-Z. Graphene-encapsulated nickel–copper bimetallic nanoparticle catalysts for electrochemical reduction of CO2 to CO. Chem. Commun. 2020, 56, 11275–11278. [Google Scholar] [CrossRef] [PubMed]
- Stephanie, R.; Kim, M.W.; Kim, S.H.; Kim, J.-K.; Park, C.Y.; Park, T.J. Recent advances of bimetallic nanomaterials and its nanocomposites for biosensing applications. TrAC Trends Anal. Chem. 2021, 135, 116159. [Google Scholar] [CrossRef]
- Su, Y.; Zheng, X.; Cheng, H.; Rao, M.; Chen, K.; Xia, J.; Lin, L.; Zhu, H. Mn-Fe3O4 nanoparticles anchored on the urushiol functionalized 3D-graphene for the electrochemical detection of 4-nitrophenol. J. Hazard. Mater. 2021, 409, 124926. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, B.; Ding, P.; Wei, W.; Ye, Y.; Wang, L.; Zhu, W.; Li, H.; Xia, J. In-situ synthesis strategy for CoM (M = Fe, Ni, Cu) bimetallic nanoparticles decorated N-doped 1D carbon nanotubes/3D porous carbon for electrocatalytic oxygen evolution reaction. J. Alloys Compd. 2020, 815, 152470. [Google Scholar] [CrossRef]
- Li, Y.; Deng, D.; Wang, H.; Huan, K.; Yan, X.; Luo, L. Controlled synthesis of Cu-Sn alloy nanosheet arrays on carbon fiber paper for self-supported nonenzymatic glucose sensing. Anal. Chim. Acta 2022, 1190, 339249. [Google Scholar] [CrossRef]
- Arikan, K.; Burhan, H.; Sahin, E.; Sen, F. A sensitive, fast, selective, and reusable enzyme-free glucose sensor based on monodisperse AuNi alloy nanoparticles on activated carbon support. Chemosphere 2021, 291, 132718. [Google Scholar] [CrossRef]
- Furong, N.; Lu, G.; Jun, A.; Yong, W. Trimetallic PdCuAu Nanoparticles for Temperature Sensing and Fluorescence Detection of H2O2 and Glucose. Front. Chem. 2020, 8, 244. [Google Scholar]
- Basavegowda, N.; Mandal, T.K.; Baek, K.H. Bimetallic and Trimetallic Nanoparticles for Active Food Packaging Applications: A Review. Food Bioprocess Technol. 2020, 13, 30–44. [Google Scholar] [CrossRef]
- Abdelwahab, A.A.; Elseman, A.M.; Alotaibi, N.F.; Nassar, A.M. Simultaneous voltammetric determination of ascorbic acid, dopamine, acetaminophen and tryptophan based on hybrid trimetallic nanoparticles-capped electropretreated grapheme. Microchem. J. 2020, 156, 104927. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, J.; Wen, Y.; Zhang, C.; Zhou, Y.; Yang, Z.; Yu, C. A trimetallic CuAuPd nanowire as a multifunctional nanocomposites applied to ultrasensitive electrochemical detection of Sema3E. Biosens. Bioelectron. 2019, 145, 111677. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, P.; Babu, R.S.; Narayanan, S.S. Electrocatalytic oxidation of L-tryptophan using copper hexacyanoferrate film modified gold nanoparticle graphite-wax electrode. Colloids Surf. B Biointerfaces 2011, 87, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Luo, L.; Ding, Y.; Liu, B.; Liu, X. Fabrication of Co3O4 nanoparticles-decorated graphene composite for determination of l-tryptophan. Analyst 2012, 137, 2840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Lu, Y.; Ding, Y.; Fu, R. An amperometric l-tryptophan sensor platform based on electrospun tricobalt tetroxide nanoparticles decorated carbon nanofibers. Sens. Actuators B Chem. 2017, 241, 601–606. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Sweena, R.; Wu, J.J. Synthesis of CuO-ZnO nanophotocatalyst for visible light assisted degradation of a textile dye in aqueous solution. Chem. Eng. J. 2011, 171, 136–140. [Google Scholar] [CrossRef]
- Kaneva, N.V.; Dimitrov, D.T.; Dushkin, C.D. Effect of nickel doping on the photocatalytic activity of ZnO thin films under UV and visible light. Appl. Surf. Sci. 2011, 257, 8113–8120. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.P.; Thakur, P. Ferromagnetism and metal-semiconducting transition in Fe-doped ZnO thin films. J. Phys. D Appl. Phys. 2008, 41, 155002. [Google Scholar] [CrossRef]
- Gacic, M.; Jakob, G.; Herbort, C. Magnetism of co-doped ZnO thin films. Phys. Rev. B 2007, 75, 205–206. [Google Scholar] [CrossRef]
- Spiridon, I.; Dascalu, I.-A.; Coroaba, A.; Apostol, I.; Palamaru, M.N.; Iordan, A.R.; Borhan, A.I. Synthesis and Characterization of New Ferrite-Lignin Hybrids. Polymers 2021, 13, 2495. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Diler, E.; Lescop, B.; Rioual, S.; Nguyen Vien, G.; Thierry, D.; Rouvellou, B. Initial formation of corrosion products on pure zinc and MgZn2 examinated by XPS. Corros. Sci. 2014, 79, 83–88. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020, 160, 696–718. [Google Scholar] [CrossRef] [PubMed]
- Tasić, Ž.Z.; Mihajlović, M.B.P.; Radovanović, M.B. Electrochemical determination of L-tryptophan in food samples on graphite electrode prepared from waste batteries. Sci. Rep. 2022, 12, 5469. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Monika, Z.; Wrona, G.D. Electrochemical oxidation of tryptophan. J. Electroanal. Chem. Interfacial Electrochem. 1986, 199, 101–126. [Google Scholar] [CrossRef]
- Pogacean, F.; Varodi, C.; Coros, M.; Kacso, I.; Radu, T.; Cozar, B.I.; Mirel, V.; Pruneanu, S. Investigation of L-Tryptophan Electrochemical Oxidation with a Graphene-Modified Electrode. Biosensors 2021, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Oliveira-Brett, A.M. Pathways of electrochemical oxidation of indolic compounds. Electroanalysis 2011, 23, 1337–1344. [Google Scholar] [CrossRef]
- Wang, Q.; Vasilescu, A.; Subramanian, P.; Vezeanu, A.; Andrei, V.; Coffinier, Y.; Li, M.; Boukherroub, R.; Szunerits, S. Simultaneous electrochemical detection of tryptophan and tyrosine using boron-doped diamond and diamond nanowire electrodes. Electrochem. Commun. 2013, 35, 84–87. [Google Scholar] [CrossRef]
- Smith, D.F. Effects of age on serum tryptophan and urine indican in adults given a tryptophan load test. Eur. J. Drug Metab. Pharmacokinet. 1982, 7, 55–58. [Google Scholar] [CrossRef]
- Yang, F. Simultaneous determination of ascorbic acid, uric acid, tryptophan and adenine using carbon-supported NiCoO2 nanoparticles. Sens. Actuator B Chem. 2015, 210, 232–240. [Google Scholar] [CrossRef]
















| Electrode | Eox (V) | Iox (µA) |
|---|---|---|
| Bare | 0.5 | 11.1 |
| ZnNp | 0.45 | 17.3 |
| CoNp | 0.4 | 18.6 |
| CuNp | 0.38 | 21.9 |
| CuZnNp | 0.35 | 25.9 |
| CuCoNp | 0.34 | 25.6 |
| CuZnCoNp | 0.26 | 37.18 |
| Electrode | Eox of Trp | Detection Technique | Sensitivity (µA/µM) | Linear Ranges (µM) | LOD (µM) | Ref |
|---|---|---|---|---|---|---|
| Flower-like CeVO4/GCE | 0.82 V | DPV | 0.0289 | 0.1–94 | 0.024 | [16] |
| CuCoHCF/graphite electrode | 0.65 V | Amperometry | 0.046 | 10–900 | 6.0 | [18] |
| Nafion/TiO2-Graphene/GCE | 0.79 V | DPV | 0.0759 | 5–140 | 0.7 | [19] |
| CeO2-RGO/GCE | 0.6 V | DPV | 0.5635 | 0.2–25 | 0.08 | [17] |
| Cu2O–ERGO/GCE | 0.92 V | SWV | 3.159 | 0.02–20 | 0.01 | [22] |
| Pd–Cu@Cu2O/N-RGO/GCE | 0.78 V | DPV | 0.3923 | 0.01–40.0 | 1.9 | [23] |
| Co3O4/Graphene/Nafion/GCE | 0.77 V | SWV | 0.1859 | 0.05–10 | 0.01 | [46] |
| NiCoO2/C modified GCE | 0.54 V | LSV | 0.0059 | 10–1000 | 1.8 | [62] |
| CuZnCo/carbon electrode | 0.26 V | DPV | 0.023 | 5–230 | 1.1 | This work |
| Milk Sample | Added Trp (µM) | Found Trp (µM) | Recovery (%) | RSD |
|---|---|---|---|---|
| 1 | 65 | 62.5 ± 0.68 | 96.1 | 5.9 |
| 2 | 110 | 104 ± 0.9 | 94.5 | 8.6 |
| Solution/Electrode | CuSO4 100 mM | ZnSO4 100 mM | CoCl2 100 mM | H2SO4 0.5 M |
|---|---|---|---|---|
| Cu | 0.5 mL | - | - | 4.5 mL |
| Zn | - | 0.5 mL | - | 4.5 mL |
| Co | - | - | 0.5 mL | 4.5 mL |
| CuZn | 0.5 mL | 0.5 mL | - | 4 mL |
| CuCo | 0.5 mL | - | 0.5 mL | 4 mL |
| CuZnCo | 0.5 mL | 0.5 mL | 0.5 mL | 3.5 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvinte, A.; Lungoci, A.-L.; Coroaba, A.; Pinteala, M. Electrochemical Sensor for Tryptophan Determination Based on Trimetallic-CuZnCo-Nanoparticle-Modified Electrodes. Molecules 2024, 29, 28. https://doi.org/10.3390/molecules29010028
Arvinte A, Lungoci A-L, Coroaba A, Pinteala M. Electrochemical Sensor for Tryptophan Determination Based on Trimetallic-CuZnCo-Nanoparticle-Modified Electrodes. Molecules. 2024; 29(1):28. https://doi.org/10.3390/molecules29010028
Chicago/Turabian StyleArvinte, Adina, Ana-Lacramioara Lungoci, Adina Coroaba, and Mariana Pinteala. 2024. "Electrochemical Sensor for Tryptophan Determination Based on Trimetallic-CuZnCo-Nanoparticle-Modified Electrodes" Molecules 29, no. 1: 28. https://doi.org/10.3390/molecules29010028
APA StyleArvinte, A., Lungoci, A. -L., Coroaba, A., & Pinteala, M. (2024). Electrochemical Sensor for Tryptophan Determination Based on Trimetallic-CuZnCo-Nanoparticle-Modified Electrodes. Molecules, 29(1), 28. https://doi.org/10.3390/molecules29010028