Dissolution of Metals (Cu, Fe, Pb, and Zn) from Different Metal-Bearing Species (Sulfides, Oxides, and Sulfates) Using Three Deep Eutectic Solvents Based on Choline Chloride
Abstract
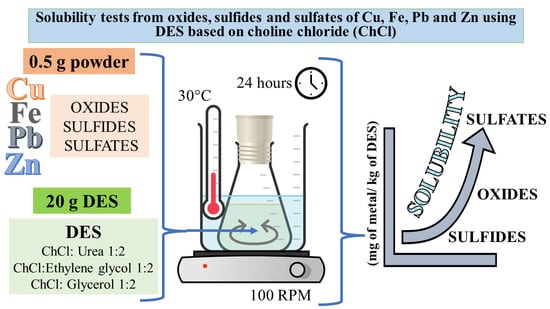
:1. Introduction
2. Results
2.1. Evaluation of the Quantity of Copper Solubilized in Deep Eutectic Solvents Based on Choline Chloride from Oxide, Sulfide, and Sulfate of This Metal
2.2. Evaluation of the Quantity of Iron Solubilized in Deep Eutectic Solvents Based on Choline Chloride from Oxide, Sulfide, and Sulfate of This Metal
Evaluation of the Quantity of Iron Solubilized in Deep Eutectic Solvents Based on Choline Chloride from Sulfides (Pyrite, Chalcopyrite, and Sphalerite)
2.3. Evaluation of the Quantity of Lead Solubilized in Deep Eutectic Solvents Based on Choline Chloride from Oxide, Sulfide, and Sulfate of This Metal
2.4. Evaluation of the Quantity of Zinc Solubilized in Deep Eutectic Solvents Based on Choline Chloride from Oxide, Sulfide, and Sulfate of This Metal
3. Discussion
Principles of the Dissolution of Metals from Sulfides, Oxides, and Sulfates in DESs (Reline, Ethaline, and Glyceline)
4. Materials and Methods
4.1. Preparation of Three Deep Eutectic Solvents Based on Choline Chloride (Reline, Ethaline, and Glyceline)
4.2. Leaching Tests of Different Metal-Bearing Species (Sulfides, Oxides, and Sulfates) Using Deep Eutectic Solvents (Reline, Ethaline, and Glyceline)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zante, G.; Boltoeva, M. Review on Hydrometallurgical Recovery of Metals with Deep Eutectic Solvents. Sustain. Chem. 2020, 1, 238–255. [Google Scholar] [CrossRef]
- Hunt, A.J.; Farmer, T.J.; Clark, J.H. Elemental Sustainability and the Importance of Scarce Element Recovery. In Element Recovery and Sustainability, 1st ed.; Hunt, A.J., Ed.; RSC Green Chemistry No. 22; Royal Society of Chemistry: London, UK, 2013; pp. 1–28. [Google Scholar] [CrossRef]
- Janssen, C.H.C.; Macías-Ruvalcaba, N.A.; Aguilar-Martínez, M.; Kobrak, M.N. Metal extraction to ionic liquids: The relationship between structure, mechanism and application. Int. Rev. Phys. Chem. 2015, 34, 591–622. [Google Scholar] [CrossRef]
- Jenkin, G.R.T.; Al-Bassam, A.Z.M.; Harris, R.C.; Abbott, A.P.; Smith, D.J.; Holwell, D.A.; Chapman, R.J.; Stanley, C.J. The application of deep eutectic solvent ionic liquids for environmentally friendly dissolution and recovery of precious metals. Miner. Eng. 2016, 87, 18–24. [Google Scholar] [CrossRef]
- Marcus, Y. Deep Eutectic Solvents; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Martins, M.; Pinho, S.; Coutinho, J. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2018, 48, 962–982. [Google Scholar] [CrossRef]
- Nolan, M.; Mezzetta, A.; Guazzelli, L.; Scanlan, E. Radical-mediated thiol–ene ‘click’ reactions in deep eutectic solvents for bioconjugation. Green Chem. 2022, 24, 1456. [Google Scholar] [CrossRef]
- Morais, E.; da Costa Lopes, A.; Freire, M.; Freire, C.; Coutinho, J.; Silvestre, A. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Li, H. Green Extraction of Polyphenols via Deep Eutectic Solvents and Assisted Technologies from Agri-Food By-Products. Molecules 2023, 28, 6852. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Mezzetta, A.; Grecchi, S.; Longhi, M.; Emanuelea, E.; Rizzoc, S.; Arduini, F.; Micheli, L.; Guazzelli, L.; Romana, P. Natural-based chiral task-specific deep eutectic solvents: A novel, effective tool for enantiodiscrimination in electroanalysis. Electrochim. Acta 2021, 380, 138189. [Google Scholar] [CrossRef]
- Abbot, A. Deep eutectic solvents and their application in electrochemistry. Curr. Opin. Green Sustain. Chem. 2022, 36, 100649. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, J.; Wang, W.; Yang, Q.; Yang, G. Deep eutectic solvents: Recent advances in fabrication approaches and pharmaceutical applications. Int. J. Pharm. 2022, 622, 121811. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of metal oxides in deep eutectic solvents based on choline chloride. J. Chem. Eng. Data 2006, 51, 1280–1282. [Google Scholar] [CrossRef]
- Abbott, A.P.; Collins, J.; Dalrymple, I.; Harris, R.C.; Mistry, R.; Qiu, F.; Scheirer, J.; Wise, W.R. Processing of Electric Arc Furnace Dust using Deep Eutectic Solvents. Aust. J. Chem. 2009, 62, 341–347. [Google Scholar] [CrossRef]
- Pateli, I.M.; Thompson, D.; Alabdullah, S.S.M.; Abbott, A.P.; Jenkin, G.R.T.; Hartley, J.M. The effect of pH and hydrogen bond donor on the dissolution of metal oxides in deep eutectic solvents. Green Chem. 2020, 22, 5476–5486. [Google Scholar] [CrossRef]
- Entezari-Zarandi, A.; Larachi, F. Selective dissolution of rare-earth element carbonates in deep eutectic solvents. J. Rare Earths 2019, 37, 528–533. [Google Scholar] [CrossRef]
- Riaño, S.; Petranikova, M.; Onghena, B.; Vander Hoogerstraete, T.; Banerjee, D.; Foreman, M.R.S.; Ekberg, C.; Binnemans, K. Separation of rare earths and other valuable metals from deepeutectic solvents: A new alternative for the recycling of used NdFeB magnets. RSC Adv. 2017, 7, 32100–32113. [Google Scholar] [CrossRef]
- Abbott, A.P.; Al-Bassam, A.Z.M.; Goddard, A.; Harris, R.C.; Jenkin, G.R.T.; Nisbet, F.J.; Wieland, M. Dissolution of pyrite and other Fe-S-As minerals using deep eutectic solvents. Green Chem. 2017, 19, 2225–2233. [Google Scholar] [CrossRef]
- Hartley, J.M.; Al-Bassam, A.Z.M.; Harris, R.C.; Frisch, G.; Jenkin, G.R.T.; Abbott, A.P. Investigating the dissolution of iron sulfide and arsenide minerals in deep eutectic solvents. Hydrometallurgy 2020, 198, 105511. [Google Scholar] [CrossRef]
- Anggara, S.; Bevan, F.; Harris, R.C.; Hartley, J.M.; Frisch, G.; Jenkin, G.R.T.; Abbott, A.P. Direct extraction of copper from copper sulfide minerals using deep eutectic solvents. Green Chem. 2019, 21, 6502–6512. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Shikotra, P. Selective Extraction of Metals from Mixed Oxide Matrixes Using Choline-Based Ionic Liquids. Inorg. Chem. 2005, 44, 6497–6499. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; Longman: Essex, UK, 1992. [Google Scholar]
- Klein, C.; Hurlbut, C.S. Manual of Mineralogy, 21st ed.; Dana, J.D., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 1937. [Google Scholar]
- Habashi, F. Handbook of Extractive Metallurgy; Wiley-VCH Verlag: Heidelberg, Germany, 1997; Volume II. [Google Scholar]
- Abbott, A.P.; Edler, K.J.; Page, A.J. Deep eutectic solvents—The vital link between ionic liquids and ionic solutions. J. Chem. Phys. 2021, 155, 150401. [Google Scholar] [CrossRef]
- Alabdullah, S.S.M. pH Measurements in Ionic Liquids. Ph.D. Thesis, University of Leicester, Leicester, UK, 2018. Available online: https://hdl.handle.net/2381/42877 (accessed on 15 November 2022).
- Binnemans, K.; Jones, P.T. Ionic Liquids and Deep-Eutectic Solvents in Extractive Metallurgy: Mismatch Between Academic Research and Industrial Applicability. J. Sustain. Metall. 2023, 9, 423–438. [Google Scholar] [CrossRef]
- Abbott, A.P.; Alabdullah, S.S.M.; Al-Murshedi, A.Y.M.; Ryder, K.S. Brønsted acidity in deep eutectic solvents and ionic liquids. Faraday Discuss. 2018, 206, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Hammond, O.; Atri, R.; Bowron, D.; de Campo, L.; Diaz-Moreno, S.; Keenan, L.; Doutch, J.; Eslava, S.; Edler, K. Structural evolution of iron forming iron oxide in a deep eutectic-solvothermal reaction. Nanoscale 2021, 13, 1723. [Google Scholar] [CrossRef]
- Carlesi, C.; Harris, R.C.; Abbott, A.P.; Jenkin, G.R.T. Chemical Dissolution of Chalcopyrite Concentrate in Choline Chloride Ethylene Glycol Deep Eutectic Solvent. Minerals 2022, 12, 65. [Google Scholar] [CrossRef]
- Isci, A.; Kaltschmitt, M. Recovery and recycling of deep eutectic solvents in biomass conversions: A review. Biomass Convers. Biorefinery 2022, 12, 197–226. [Google Scholar] [CrossRef]
- Delso, I.; Lafuente, C.; Muñoz-Embid, J.; Artal, M. NMR study of choline chloride-based deep eutectic solvents. J. Mol. Liq. 2019, 290, 111236. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. The Twelve Principles of Circular Hydrometallurgy. J. Sustain. Metall. 2022, 9, 1–25. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragón-Tobar, C.F.; Endara, D.; de la Torre, E. Dissolution of Metals (Cu, Fe, Pb, and Zn) from Different Metal-Bearing Species (Sulfides, Oxides, and Sulfates) Using Three Deep Eutectic Solvents Based on Choline Chloride. Molecules 2024, 29, 290. https://doi.org/10.3390/molecules29020290
Aragón-Tobar CF, Endara D, de la Torre E. Dissolution of Metals (Cu, Fe, Pb, and Zn) from Different Metal-Bearing Species (Sulfides, Oxides, and Sulfates) Using Three Deep Eutectic Solvents Based on Choline Chloride. Molecules. 2024; 29(2):290. https://doi.org/10.3390/molecules29020290
Chicago/Turabian StyleAragón-Tobar, Carlos F., Diana Endara, and Ernesto de la Torre. 2024. "Dissolution of Metals (Cu, Fe, Pb, and Zn) from Different Metal-Bearing Species (Sulfides, Oxides, and Sulfates) Using Three Deep Eutectic Solvents Based on Choline Chloride" Molecules 29, no. 2: 290. https://doi.org/10.3390/molecules29020290
APA StyleAragón-Tobar, C. F., Endara, D., & de la Torre, E. (2024). Dissolution of Metals (Cu, Fe, Pb, and Zn) from Different Metal-Bearing Species (Sulfides, Oxides, and Sulfates) Using Three Deep Eutectic Solvents Based on Choline Chloride. Molecules, 29(2), 290. https://doi.org/10.3390/molecules29020290