Towards Industrially Important Applications of Enhanced Organic Reactions by Microfluidic Systems
Abstract
:1. Introduction
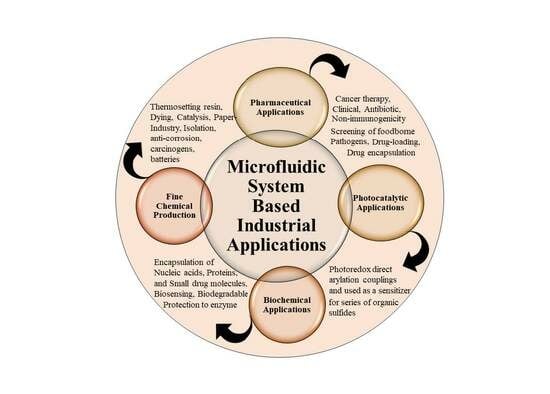
2. Industrial Applications and Microfluidic Synthesis
2.1. Pharmaceutical Applications
2.2. Photocatalytic Applications
2.3. Biochemical Applications
2.4. Fine Chemical Production
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nogala, W.; Szot, K.; Burchardt, M.; Roelfs, F.; Rogalski, J.; Opallo, M.; Wittstock, G. Feedback mode SECM study of laccase and bilirubin oxidase immobilised in a sol–gel processed silicate film. Analyst 2010, 135, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Ristenpart, W.D.; Wan, J.; Stone, H.A. Enzymatic reactions in microfluidic devices: Michaelis−Menten kinetics. Anal. Chem. 2008, 80, 3270–3276. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Ikeda, S.; Tanaka, J.; Go, J.S.; Nakanishi, H.; Shoji, S. The multiple sample injector using improved sheath flow to prevent sample dilution. Sens. Actuators A Phys. 2004, 111, 32–36. [Google Scholar] [CrossRef]
- Atencia, J.; Beebe, D.J. Controlled microfluidic interfaces. Nature 2005, 437, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Jamshaid, A.; Ito, J.; Nakahara, A.; Tanaka, D.; Akitsu, T.; Sekiguchi, T.; Shoji, S. Active microdroplet merging by hydrodynamic flow control using a pneumatic actuator-assisted pillar structure. Lab Chip 2014, 14, 3050–3055. [Google Scholar] [CrossRef]
- Neumann, M.; Zeitler, K. Application of Microflow Conditions to Visible Light Photoredox Catalysis. Org. Lett. 2012, 14, 2658–2661. [Google Scholar] [CrossRef]
- Trojanowicz, M. Flow chemistry in contemporary chemical sciences: A real variety of its applications. Molecules 2020, 25, 1434. [Google Scholar] [CrossRef]
- Fukuda, T.; Funaki, N.; Kurabayashi, T.; Suzuki, M.; Yoon, D.H.; Nakahara, A.; Sekiguchi, T.; Shoji, S. Real-time monitoring of chemical reaction in microdroplet using fluorescence spectroscopy. Sens. Actuators B Chem. 2014, 203, 536–542. [Google Scholar] [CrossRef]
- Kang, J.; Lhee, S.; Lee, J.K.; Zare, R.N.; Nam, H.G. Restricted intramolecular rotation of fluorescent molecular rotors at the periphery of aqueous microdroplets in oil. Sci. Rep. 2020, 10, 16859. [Google Scholar] [CrossRef]
- Tanaka, D.; Kawakubo, W.; Tsuda, E.; Mitsumoto, Y.; Yoon, D.H.; Sekiguchi, T.; Akitsu, T.; Shoji, S. Microfluidic synthesis of chiral salen Mn (ii) and Co (ii) complexes containing lysozyme. RSC Adv. 2016, 6, 81862–81868. [Google Scholar] [CrossRef]
- Brivio, M.; Verboom, W.; Reinhoudt, D.N. Miniaturized continuous flow reaction vessels: Influence on chemical reactions. Lab Chip 2006, 6, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Gnanamani, E.; Yan, X.; Zare, R.N. Can all bulk-phase reactions be accelerated in microdroplets? Analyst 2017, 142, 1399–1402. [Google Scholar] [CrossRef]
- Priest, C.; Zhou, J.; Klink, S.; Sedev, R.; Ralston, J. Microfluidic solvent extraction of metal ions and complexes from leach solutions containing nanoparticles. Chem. Eng. Technol. 2012, 35, 1312–1319. [Google Scholar] [CrossRef]
- Tuck, A.F. Gibbs free energy and reaction rate acceleration in and on microdroplets. Entropy 2019, 21, 1044. [Google Scholar] [CrossRef]
- Tanaka, D.; Sawai, S.; Yoon, D.H.; Sekiguchi, T.; Akitsu, T.; Shoji, S. Synthesis of an azo-Mn (II) complex with mild pH control using a microfluidic device. RSC Adv. 2017, 7, 39576–39582. [Google Scholar] [CrossRef]
- Fukuyama, M.; Hibara, A.; Yoshida, Y.; Maeda, K. Kinetic switching of the concentration/separation behavior of microdroplets. Anal. Chem. 2017, 89, 9279–9283. [Google Scholar] [CrossRef] [PubMed]
- Nicole, P. Comparison of Photo-oxidation Reactions in Batch and a New Photosensitizer-Immobilized Microfluidic Device. Org. Lett. 2012, 14, 5724–5727. [Google Scholar]
- Kim, H.; Min, K.-I.; Inoue, K.; Im, D.J.; Kim, D.-P.; Yoshida, J.-I. Submillisecond organic synthesis: Outpacing Fries rearrangement through microfluidic rapid mixing. Science 2016, 352, 691–694. [Google Scholar] [CrossRef]
- Kobayashi, M.; Akitsu, T.; Furuya, M.; Sekiguchi, T.; Shoji, S.; Tanii, T.; Tanaka, D. Efficient Synthesis of a Schiff Base Copper (II) Complex Using a Microfluidic Device. Micromachines 2023, 14, 890. [Google Scholar] [CrossRef]
- Coats, J.P.; Cochereau, R.; Dinu, I.A.; Messmer, D.; Sciortino, F.; Palivan, C.G. Trends in the synthesis of polymer nano-and microscale materials for bio-related applications. Macromol. Biosci. 2023, 23, 2200474. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Zhang, W.; Hu, W.; Wang, Y.; Wu, J.; Zhu, D.; Wang, Q.; Shi, M.; Yan, X. Inertial migration of fine mineral particles in a curved microfluidic channel: Demystifying the role of non-neutrally buoyant particles. Sep. Purif. Technol. 2023, 334, 126026. [Google Scholar] [CrossRef]
- Long, F.; Guo, Y.; Zhang, Z.; Wang, J.; Ren, Y.; Cheng, Y.; Xu, G. Recent Progress of Droplet Microfluidic Emulsification Based Synthesis of Functional Microparticles. Glob. Chall. 2023, 7, 2300063. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, T.; Tanaka, D.; Yoon, D.H.; Nozaki, Y.; Sekiguchi, T.; Akitsu, T.; Shoji, S. High-Efficiency Dibromination of Organic Compound in Microfluidic Channel of SI Pillar Array Directly Coated with Iron Catalys. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 2278–2281. [Google Scholar]
- Wei, Y.; Ma, G.; Zhang, H.; Zhang, X.; Quan, X. Optimization design of optical windows for deep-sea pressure-resistant structures based on transition materials. Front. Mar. Sci. 2023, 10, 1211337. [Google Scholar] [CrossRef]
- Sharma, M.K.; Khan, M.S.; Kulkarni, A.A. Flow Distribution of Multiphase Flow in Parallel Channels. In Handbook of Multiphase Flow Science and Technology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1241–1277. [Google Scholar]
- Zhao, R.; Tan, C.; Xie, Y.; Gao, C.; Liu, H.; Jiang, Y. One step synthesis of azo compounds from nitroaromatics and anilines. Tetrahedron Lett. 2011, 52, 3805–3809. [Google Scholar] [CrossRef]
- Ashraf, S.; Liu, Y.; Wei, H.; Shen, R.; Zhang, H.; Wu, X.; Mehdi, S.; Liu, T.; Li, B. Bimetallic Nanoalloy Catalysts for Green Energy Production: Advances in Synthesis Routes and Characterization Techniques. Small 2023, 19, e2303031. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Yin, J.; Wang, H.; Liu, B.; Bennacer, R. Experimental study on the evaporating progress of hexane lens on immiscible liquid: Spreading and receding. E3S Web Conf. 2021, 321, 01017. [Google Scholar] [CrossRef]
- Liu, D.; Wang, K.; Wang, Y.; Wang, Y.; Luo, G. A simple online phase separator for the microfluidic mass transfer studies. Chem. Eng. J. 2017, 325, 342–349. [Google Scholar] [CrossRef]
- He, S.; Joseph, N.; Feng, S.; Jellicoe, M.; Raston, C.L. Application of microfluidic technology in food processing. Food Funct. 2020, 11, 5726–5737. [Google Scholar] [CrossRef]
- Neethirajan, S.; Kobayashi, I.; Nakajima, M.; Wu, D.; Nandagopal, S.; Lin, F. Microfluidics for food, agriculture and biosystems industries. Lab Chip 2011, 11, 1574–1586. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Iakovlev, A.P.; Erofeev, A.S.; Gorelkin, P.V. Novel pumping methods for microfluidic devices: A comprehensive review. Biosensors 2022, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Zhang, N. Perspectives in translating microfluidic devices from laboratory prototyping into scale-up production. Biomicrofluidics 2022, 16, 021301. [Google Scholar] [CrossRef] [PubMed]
- Forigua, A.; Kirsch, R.L.; Willerth, S.M.; Elvira, K.S. Recent advances in the design of microfluidic technologies for the manufacture of drug releasing particles. J. Control. Release 2021, 333, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-J.; Coley, C.W.; Abolhasani, M.; Marzinzik, A.L.; Koch, G.; Spanka, C.; Lehmann, H.; Jensen, K.F. A segmented flow platform for on-demand medicinal chemistry and compound synthesis in oscillating droplets. Chem. Commun. 2017, 53, 6649–6652. [Google Scholar] [CrossRef]
- Beulig, R.; Warias, R.; Heiland, J.; Ohla, S.; Zeitler, K.; Belder, D. A droplet-chip/mass spectrometry approach to study organic synthesis at nanoliter scale. Lab Chip 2017, 17, 1996–2002. [Google Scholar] [CrossRef]
- Sun, A.C.; Steyer, D.J.; Allen, A.R.; Payne, E.M.; Kennedy, R.T.; Stephenson, C.R. A droplet microfluidic platform for high-throughput photochemical reaction discovery. Nat. Commun. 2020, 11, 6202. [Google Scholar] [CrossRef]
- Mo, Y.; Lu, Z.; Rughoobur, G.; Patil, P.; Gershenfeld, N.; Akinwande, A.I.; Buchwald, S.L.; Jensen, K.F. Microfluidic electrochemistry for single-electron transfer redox-neutral reactions. Science 2020, 368, 1352–1357. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, B.; Zhang, D.; Liu, Y.; Zhang, M.; Zhao, C.; Zheng, J. Design principles and fundamental understanding of biosensors for amyloid-β detection. J. Mater. Chem. B 2020, 8, 6179–6196. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Koklu, A.; Wustoni, S.; Musteata, V.-E.; Ohayon, D.; Moser, M.; McCulloch, I.; Nunes, S.P.; Inal, S. Microfluidic integrated organic electrochemical transistor with a nanoporous membrane for amyloid-β detection. ACS Nano 2021, 15, 8130–8141. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Jaouhari, T.; Zhang, F.; Tassaing, T.; Fery-Forgues, S.; Aymonier, C.; Marre, S.; Erriguible, A. Process intensification for the synthesis of ultra-small organic nanoparticles with supercritical CO2 in a microfluidic system. Chem. Eng. J. 2020, 397, 125333. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2 (H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Koryakina, I.G.; Bachinin, S.V.; Gerasimova, E.N.; Timofeeva, M.V.; Shipilovskikh, S.A.; Bukatin, A.S.; Sakhatskii, A.; Timin, A.S.; Milichko, V.A.; Zyuzin, M.V. Microfluidic synthesis of metal-organic framework crystals with surface defects for enhanced molecular loading. Chem. Eng. J. 2023, 452, 139450. [Google Scholar] [CrossRef]
- Shen, J.; Ma, M.; Zhang, H.; Yu, H.; Xue, F.; Hao, N.; Chen, H. Microfluidics-assisted surface trifunctionalization of a zeolitic imidazolate framework nanocarrier for targeted and controllable multitherapies of tumors. ACS Appl. Mater. Interfaces 2020, 12, 45838–45849. [Google Scholar] [CrossRef]
- Silva, N.F.; Magalhães, J.M.; Freire, C.; Delerue-Matos, C. Electrochemical biosensors for Salmonella: State of the art and challenges in food safety assessment. Biosens. Bioelectron. 2018, 99, 667–682. [Google Scholar] [CrossRef]
- Kong, R.; Lee, S.; Law, T.; Law, S.; Wu, R. Rapid detection of six types of bacterial pathogens in marine waters by multiplex PCR. Water Res. 2002, 36, 2802–2812. [Google Scholar] [CrossRef]
- Sayad, A.; Ibrahim, F.; Uddin, S.M.; Cho, J.; Madou, M.; Thong, K.L. A microdevice for rapid, monoplex and colorimetric detection of foodborne pathogens using a centrifugal microfluidic platform. Biosens. Bioelectron. 2018, 100, 96–104. [Google Scholar] [CrossRef]
- Tian, F.; Lyu, J.; Shi, J.; Tan, F.; Yang, M. A polymeric microfluidic device integrated with nanoporous alumina membranes for simultaneous detection of multiple foodborne pathogens. Sens. Actuators B Chem. 2016, 225, 312–318. [Google Scholar] [CrossRef]
- Qi, W.; Zheng, L.; Wang, S.; Huang, F.; Liu, Y.; Jiang, H.; Lin, J. A microfluidic biosensor for rapid and automatic detection of Salmonella using metal-organic framework and Raspberry Pi. Biosens. Bioelectron. 2021, 178, 113020. [Google Scholar] [CrossRef]
- Chandel, A.K.; Rao, L.V.; Narasu, M.L.; Singh, O.V. The realm of penicillin G acylase in β-lactam antibiotics. Enzym. Microb. Technol. 2008, 42, 199–207. [Google Scholar] [CrossRef]
- Vobecká, L.; Tichá, L.; Atanasova, A.; Slouka, Z.; Hasal, P.; Přibyl, M. Enzyme synthesis of cephalexin in continuous-flow microfluidic device in ATPS environment. Chem. Eng. J. 2020, 396, 125236. [Google Scholar] [CrossRef]
- Chen, W.; Yang, W.; Chen, P.; Huang, Y.; Li, F. Disulfiram copper nanoparticles prepared with a stabilized metal ion ligand complex method for treating drug-resistant prostate cancers. ACS Appl. Mater. Interfaces 2018, 10, 41118–41128. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jiang, J.; Chen, W.; Yang, W.; Chen, L.; Chen, P.; Shen, J.; Qian, S.; Zhou, T.; Wu, L. Biomimetic metal-organic nanoparticles prepared with a 3D-printed microfluidic device as a novel formulation for disulfiram-based therapy against breast cancer. Appl. Mater. Today 2020, 18, 100492. [Google Scholar] [CrossRef] [PubMed]
- Arduino, I.; Liu, Z.; Rahikkala, A.; Figueiredo, P.; Correia, A.; Cutrignelli, A.; Denora, N.; Santos, H.A. Preparation of cetyl palmitate-based PEGylated solid lipid nanoparticles by microfluidic technique. Acta Biomater. 2021, 121, 566–578. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, Y.; Li, B.; Qi, W.; Su, R.; He, Z. Microfluidic synthesis of lignin/chitosan nanoparticles for the pH-responsive delivery of anticancer drugs. Langmuir 2021, 37, 7219–7226. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, K.; Rao, K.M.; Sudhakar, P.; ChandraBabu, A.; Kumara Babu, P.; Subha, M.; Chowdoji Rao, K. Development of pH-sensitive polycaprolactone-based microspheres for in vitro release studies of Triprolidine Hydrochloride. Des. Monomers Polym. 2014, 17, 617–623. [Google Scholar] [CrossRef]
- Tavana, B.; Khatibi, A.; Jafarkhani, S.; Zahedi, P.; Zamani, M.H.; Jafari, S.H.; Najafi, M. Simulation and in vitro evaluations of microfluidically-fabricated clarithromycin-poly (ε-caprolactone) nanoparticles. J. Ind. Eng. Chem. 2023, 124, 211–223. [Google Scholar] [CrossRef]
- Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S.D.; Noël, T. Technological innovations in photochemistry for organic synthesis: Flow chemistry, high-throughput experimentation, scale-up, and photoelectrochemistry. Chem. Rev. 2021, 122, 2752–2906. [Google Scholar] [CrossRef]
- Hone, C.A.; Kappe, C.O. Towards the standardization of flow chemistry protocols for organic reactions. Chem.-Methods 2021, 1, 454–467. [Google Scholar] [CrossRef]
- Dinter, R.; Willems, S.; Nissalk, T.; Hastürk, O.; Brunschweiger, A.; Kockmann, N. Development of a microfluidic photochemical flow reactor concept by rapid prototyping. Front. Chem. 2023, 11, 1244043. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-Y.; Chiou, Y.-R.; Luo, R.-H.; Chen, T.-H.; Yu, C.-J.; Chou, Y.-J.; Chang, H.-T.; Chen, C.-F. Partially miscible droplet microfluidics to enhance interfacial adsorption of hydrophilic nanoparticles for colloidosome synthesis. Chem. Eng. J. 2023, 471, 144223. [Google Scholar] [CrossRef]
- Pallini, F.; Sangalli, E.; Sassi, M.; Roth, P.M.; Mattiello, S.; Beverina, L. Selective photoredox direct arylations of aryl bromides in water in a microfluidic reactor. Org. Biomol. Chem. 2021, 19, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Shen, Y.; Lignos, I.; Bawendi, M.G.; Jensen, K.F. Multistage microfluidic platform for the continuous synthesis of III–V core/shell quantum dots. Angew. Chem. Int. Ed. 2018, 57, 10915–10918. [Google Scholar] [CrossRef]
- Chowdhury, M.S.; Zheng, W.; Kumari, S.; Heyman, J.; Zhang, X.; Dey, P.; Weitz, D.A.; Haag, R. Dendronized fluorosurfactant for highly stable water-in-fluorinated oil emulsions with minimal inter-droplet transfer of small molecules. Nat. Commun. 2019, 10, 4546. [Google Scholar] [CrossRef] [PubMed]
- Jayamohan, H.; Smith, Y.R.; Gale, B.K.; Mohanty, S.K.; Misra, M. Photocatalytic microfluidic reactors utilizing titania nanotubes on titanium mesh for degradation of organic and biological contaminants. J. Environ. Chem. Eng. 2016, 4, 657–663. [Google Scholar] [CrossRef]
- Li, J.; An, Z.; Sun, J.; Tan, C.; Gao, D.; Tan, Y.; Jiang, Y. Highly selective oxidation of organic sulfides by a conjugated polymer as the photosensitizer for singlet oxygen generation. ACS Appl. Mater. Interfaces 2020, 12, 35475–35481. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.-T.; Sun, L.; Shiu, B.-C.; Zhang, L.; Lin, J.-H.; Lou, C.-W. Oriented ascorbic acid onto zeolitic metal-organic framework-8 membrane via microfluidic spinning for biomedical care. Colloids Surf. B Biointerfaces 2023, 229, 113442. [Google Scholar] [CrossRef]
- Rohra, N.; Gaikwad, G.; Dandekar, P.; Jain, R. Microfluidic Synthesis of a Bioactive Metal–Organic Framework for Glucose-Responsive Insulin Delivery. ACS Appl. Mater. Interfaces 2022, 14, 8251–8265. [Google Scholar] [CrossRef]
- Hu, C.; Bai, Y.; Hou, M.; Wang, Y.; Wang, L.; Cao, X.; Chan, C.-W.; Sun, H.; Li, W.; Ge, J. Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis. Sci. Adv. 2020, 6, eaax5785. [Google Scholar] [CrossRef]
- Lian, J.; Ozga, A.J.; Sokol, C.L.; Luster, A.D. Targeting lymph node niches enhances type 1 immune responses to immunization. Cell Rep. 2020, 31, 107679. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, H.; Liu, Y.; Mao, L.; Chen, W.; Zhu, Z.; Liu, W.; Zheng, W.; Zhao, Y.; Kong, D. A peptide-based nanofibrous hydrogel as a promising DNA nanovector for optimizing the efficacy of HIV vaccine. Nano Lett. 2014, 14, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Hong, H.; Lee, Y.; Park, K.S.; Sun, M.; Wang, T.; Aikins, M.E.; Xu, Y.; Moon, J.J. Efficient lymph node-targeted delivery of personalized cancer vaccines with reactive oxygen species-inducing reduced graphene oxide nanosheets. ACS Nano 2020, 14, 13268–13278. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, Y.L.; Li, X.; Jiang, X. Integrated microfluidic synthesis of aptamer functionalized biozeolitic imidazolate framework (BioZIF-8) targeting lymph node and tumor. Nano Lett. 2021, 21, 1335–1344. [Google Scholar] [CrossRef]
- Delama, A.; Teixeira, M.I.; Dorati, R.; Genta, I.; Conti, B.; Lamprou, D.A. Microfluidic encapsulation method to produce stable liposomes containing iohexol. J. Drug Deliv. Sci. Technol. 2019, 54, 101340. [Google Scholar] [CrossRef]
- Ballacchino, G.; Weaver, E.; Mathew, E.; Dorati, R.; Genta, I.; Conti, B.; Lamprou, D.A. Manufacturing of 3D-printed microfluidic devices for the synthesis of drug-loaded liposomal formulations. Int. J. Mol. Sci. 2021, 22, 8064. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Maeki, M.; Sato, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of a microfluidic-based post-treatment process for size-controlled lipid nanoparticles and application to siRNA delivery. ACS Appl. Mater. Interfaces 2020, 12, 34011–34020. [Google Scholar] [CrossRef]
- Sato, Y.; Note, Y.; Maeki, M.; Kaji, N.; Baba, Y.; Tokeshi, M.; Harashima, H. Elucidation of the physicochemical properties and potency of siRNA-loaded small-sized lipid nanoparticles for siRNA delivery. J. Control. Release 2016, 229, 48–57. [Google Scholar] [CrossRef]
- Mao, C.; Wang, S.; Li, J.; Feng, Z.; Zhang, T.; Wang, R.; Fan, C.; Jiang, X. Metal–Organic Frameworks in Microfluidics Enable Fast Encapsulation/Extraction of DNA for Automated and Integrated Data Storage. ACS Nano 2023, 17, 2840–2850. [Google Scholar] [CrossRef]
- Akitsu, T.; Tanaka, R. Polarized Electronic and IR Spectra of Hybrid Materials of Chiral Mn (II) Complexes and Different Types of Photochromic Dyes Showing Photoisomerization or Weigert Effect. Curr. Phys. Chem. 2011, 1, 82–89. [Google Scholar] [CrossRef]
- Stastna, M. Continuous flow electrophoretic separation—Recent developments and applications to biological sample analysis. Electrophoresis 2020, 41, 36–55. [Google Scholar] [CrossRef]
- Aritake, Y.; Takanashi, T.; Yamazaki, A.; Akitsu, T. Polarized spectroscopy and hybrid materials of chiral Schiff base Ni (II), Cu (II), Zn (II) complexes with included or separated azo-groups. Polyhedron 2011, 30, 886–894. [Google Scholar] [CrossRef]
- Tanaka, D.; Sawai, S.; Hattori, S.; Nozaki, Y.; Yoon, D.H.; Fujita, H.; Sekiguchi, T.; Akitsu, T.; Shoji, S. Microdroplet synthesis of azo compounds with simple microfluidics-based pH control. RSC Adv. 2020, 10, 38900–38905. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.N.; Taploo, C. Schiff bases and their applications. J. Sci. Ind. Res. 1982, 41, 501–506. [Google Scholar]
- Abd El-Lateef, H.M.; Abu-Dief, A.M.; Mohamed, M.A. Corrosion inhibition of carbon steel pipelines by some novel Schiff base compounds during acidizing treatment of oil wells studied by electrochemical and quantum chemical methods. J. Mol. Struct. 2017, 1130, 522–542. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Dabea, T.; Khalaf, M.M.; Abu-Dief, A.M. Innovation of imine metal chelates as corrosion inhibitors at different media: A collective study. Int. J. Mol. Sci. 2022, 23, 9360. [Google Scholar] [CrossRef]
- Ghanghas, P.; Choudhary, A.; Kumar, D.; Poonia, K. Coordination metal complexes with Schiff bases: Useful pharmacophores with comprehensive biological applications. Inorg. Chem. Commun. 2021, 130, 108710. [Google Scholar] [CrossRef]
- Khanam, W.; Dubey, N. Recent advances in immobilized ω-transaminase for chiral amine synthesis. Mater. Today Chem. 2022, 24, 100922. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. 2008, 120, 8958–8962. [Google Scholar] [CrossRef]
- Spitler, E.L.; Dichtel, W.R. Lewis acid-catalysed formation of two-dimensional phthalocyanine covalent organic frameworks. Nat. Chem. 2010, 2, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-X.; Cooper, A.I. Microporous organic polymers: Design, synthesis, and function. In Functional Metal-Organic Frameworks: Gas Storage, Separation and Catalysis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–33. [Google Scholar]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Colson, J.W.; Dichtel, W.R. Rationally synthesized two-dimensional polymers. Nat. Chem. 2013, 5, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-San-Miguel, D.; Abrishamkar, A.; Navarro, J.A.; Rodriguez-Trujillo, R.; Amabilino, D.B.; Mas-Ballesté, R.; Zamora, F.; Puigmartí-Luis, J. Crystalline fibres of a covalent organic framework through bottom-up microfluidic synthesis. Chem. Commun. 2016, 52, 9212–9215. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wu, M.; Dong, K.; Zhang, M.; Cheng, Y.; Shi, G. Highly efficient synthesis and application of aryl diazonium salts via femtosecond laser-tailored 3D flow microfluidic chips. Chin. Chem. Lett. 2023, 34, 107694. [Google Scholar] [CrossRef]
- Jankowski, P.; Kutaszewicz, R.; Ogończyk, D.; Garstecki, P. A microfluidic platform for screening and optimization of organic reactions in droplets. J. Flow Chem. 2020, 10, 397–408. [Google Scholar] [CrossRef]
- Ren, J.; Wang, F.; Wu, M.; Cheng, Y.; Shi, G. A high-throughput femtosecond laser-engineered 3D microfluidic chip for efficient chemical transformation of carbon dioxide to value-added chemicals. Chem. Eng. J. 2023, 476, 146758. [Google Scholar] [CrossRef]
- Fath, V.; Kockmann, N.; Otto, J.; Röder, T. Self-optimising processes and real-time-optimisation of organic syntheses in a microreactor system using Nelder–Mead and design of experiments. React. Chem. Eng. 2020, 5, 1281–1299. [Google Scholar] [CrossRef]
- Pilato, L. Phenolic resins: 100 Years and still going strong. React. Funct. Polym. 2013, 73, 270–277. [Google Scholar] [CrossRef]
- Foyer, G.; Chanfi, B.-H.; Boutevin, B.; Caillol, S.; David, G. New method for the synthesis of formaldehyde-free phenolic resins from lignin-based aldehyde precursors. Eur. Polym. J. 2016, 74, 296–309. [Google Scholar] [CrossRef]
- Yi, T.; Zhao, S.; Gao, W.; Guo, C.; Yang, L.; Du, G. The similar in-situ polymerization of nano cupric oxide preparation and phenol formaldehyde resin synthesis: The process and mechanism. Int. J. Adhes. Adhes. 2018, 87, 109–118. [Google Scholar] [CrossRef]
- Babu, T.G.; Bhuvaneswari, S.; Devasia, R. Synthesis and ceramic conversion of novel silazane modified phenol formaldehyde resin. Mater. Chem. Phys. 2018, 212, 175–186. [Google Scholar] [CrossRef]
- Qiao, W.; Li, S.; Guo, G.; Han, S.; Ren, S.; Ma, Y. Synthesis and characterization of phenol-formaldehyde resin using enzymatic hydrolysis lignin. J. Ind. Eng. Chem. 2015, 21, 1417–1422. [Google Scholar] [CrossRef]
- Kałȩdkowski, B.; Hetper, J. Synthesis of phenol–formaldehyde resole resins in the presence of tetraalkylammonium hydroxides as catalysts. Polymer 2000, 41, 1679–1684. [Google Scholar] [CrossRef]
- Yu, Q.; Guan, D.; Zhuang, Z.; Li, J.; Shi, C.; Luo, W.; Zhou, L.; Zhao, D.; Mai, L. Mass production of monodisperse carbon microspheres with size-dependent supercapacitor performance via aqueous self-catalyzed polymerization. ChemPlusChem 2017, 82, 872–878. [Google Scholar] [CrossRef]
- Liu, F.; Yin, K.; Liu, H. A microfluidic synthesis method for preparation and regulation of 3-aminophenol formaldehyde resin spheres. React. Funct. Polym. 2021, 165, 104973. [Google Scholar] [CrossRef]





















| Experiments | Type of Reaction | Advantages | Pharmaceutical Applications |
|---|---|---|---|
| Electrosynthesis of nematic liquid crystal compounds | Microfluidic redox-neutral molecular diffusion | Enhanced reactivity, Handle several samples simultaneously over a long period, Rapid molecular diffusion | Facilitates selective transformation |
| Detect amyloid-β protein in human serum | Microfluidic organic electrochemical transistors reaction | Reduced analytical time, Minimal sample, Not required reference electrodes | Cure Alzheimer’s disease |
| Organic TPE nanoparticles (NPs) | Microreactor based antisolvent process | Possibility to obtain sizes below 15 nm | Imaging, biosensing and medicine |
| MOF microcrystals with HKUST-1 | Continuous flow synthesis in a microfluidic chip | 2 times higher loading capacities than a solvothermally synthesized compound | Utilize them as drug delivery systems |
| Zeolitic imidazolate framework-90 (ZIF-90) core and a SAD shell | Microfluidics-Assisted Surface Trifunctionalization | 10-fold loading capacity over ZIF-90 obtained by conventional means, prevent drug leakage | Cancer therapy, clinical applications |
| 2,3-diaminophenazine (DAP) production | Used microfluidic biosensors | Greater sensitivity, improved image processing. rapid mixing | In-field screening of foodborne pathogens |
| Cephalexin is synthesized by penicillin acylase | Microfluidic aqueous two-phase system | High affinity, allows simple enzyme recycle and addition of fresh reactants. | Used as antibiotic |
| Solid-Lipid Nanoparticles (SLNs) | Oil-in-water homogenization microfluidic process | High yields and highly reproducible | Good encapsulation efficiency and loading degree of the drugs |
| PCL nanoparticles containing clarithromycin | Microfluidic hydrodynamic flow focusing reaction | NPs were smaller, more uniform, and more stable than the NPs prepared using conventional methods | Significant drug-loading capacity, biodegradability, non-immunogenicity |
| Bovine serum albumin production | Scalable continuous microfluidic assisted SMILE technology | Precise mixing control, easily scale up preparation for mass production | Potential for treating breast cancer |
| Experiments | Type of Reaction | Advantages | Photocatalytic Applications |
|---|---|---|---|
| Arylated compounds | Photoredox reaction | Improves the reaction time, without eroding the yields and selectivity | Carrying out photoredox direct arylation couplings |
| Cationic poly(p-phenylene ethynylene terthiophene) | Photocatalytic oxidation | Transfer heat and mass rapidly, have short molecular diffusion distances and easy to control | Used as a sensitizer for series of organic sulfides |
| Experiments | Type of Reaction | Advantages | Biochemical applications |
|---|---|---|---|
| A zeolitic metal-organic framework-8 at ascorbic acid | Electrostatic hydrogen bonding, microfluidic spinning | Excellent biocompatibility and hemocompatibility | Construction of multifunctional wound healing materials |
| Insulin- and AuNP-encapsulated zeolitic imidazolate framework-8 | Continuous-flow, microfluidic oxidation system | Due to presence of glucose stimulus for insulin release | Drug delivery, biosensing applications |
| Enzyme-MOF composites | Microfluidic laminar flow | Reducing resistance to mass transfer | Maintaining the protection to the enzymes |
| BioZIF-8 MOFs with an aptamer | Electrostatic one chip microfluidic reaction | Lower toxicity and more effective at targeting lymph nodes and tumor mass | Encapsulation of nucleic acids, proteins, and small drug molecules |
| Curcumin-loaded Liposomes | Microfluidic polymerization | Controlling the channel length, flow rates, composition, pH and temperature | Anticancer activity |
| Small interfering RNA-loaded LNPs | Continuous-flow, microfluidic system | High knockdown activity and low accumulation | Used for the treatment of various cancers |
| Chiral salen Mn(II) and Co(II) complexes with lysozyme | Microfluidic oxidation reaction | Reaction time would be lower at lower temperatures | Used in drugdiscovery or fuel cells |
| Experiments | Type of Reaction | Advantages | Fine chemical Applications |
|---|---|---|---|
| Azo compounds synthesis | Diazotization reaction | pH control, short reaction time, at room temperature | Batteries and anticancer medications |
| Cu(II) complexes with a Schiff base | Laminar flow microfluidic reaction | Lower reagent quantity, precise control of the flow rate, reaction performance 700 times greater than conventional method | High biological activity and catalytic function, anti-corrosion, dyes, pigments, antioxidants, carcinogens, and antimicrobial agents |
| Imine-based COF material (MF-COF-1) | Self-condensation reactions, | At atm pressure and room temperature, compound manufactured in only a few seconds | Direct drawing of objects on surface |
| Arylated N-methyl pyrrole with ethyl-4-bromobenzoate | Microfluidic arylation reaction | Provide high surface-to-volume ratio | Polymer chemistry, biomedical science, pharmacy, isolation, and photochromism |
| Phenolic resins | Oil in water microfluidic reaction | Controlled residence time and flow rate | Thermosetting resin |
| Continuous CO2 transformation in multiphase flow | Gas-liquid carboxylation | High yield and short reaction time | Replacing the use of fossil fuel resources |
| Imine formation-(E)-1-(2-nitrophenyl)-N-phenethylmethanimine | Microfluidic condensation reaction | Low ratio of reagents, temperature, and reaction time | Dying, catalysis, paper industry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, A.; Takeda, C.; Manzoor, A.; Tanaka, D.; Kobayashi, M.; Wadayama, Y.; Nakane, D.; Majeed, A.; Iqbal, M.A.; Akitsu, T. Towards Industrially Important Applications of Enhanced Organic Reactions by Microfluidic Systems. Molecules 2024, 29, 398. https://doi.org/10.3390/molecules29020398
Zafar A, Takeda C, Manzoor A, Tanaka D, Kobayashi M, Wadayama Y, Nakane D, Majeed A, Iqbal MA, Akitsu T. Towards Industrially Important Applications of Enhanced Organic Reactions by Microfluidic Systems. Molecules. 2024; 29(2):398. https://doi.org/10.3390/molecules29020398
Chicago/Turabian StyleZafar, Ayesha, China Takeda, Asif Manzoor, Daiki Tanaka, Masashi Kobayashi, Yoshitora Wadayama, Daisuke Nakane, Adnan Majeed, Muhammad Adnan Iqbal, and Takashiro Akitsu. 2024. "Towards Industrially Important Applications of Enhanced Organic Reactions by Microfluidic Systems" Molecules 29, no. 2: 398. https://doi.org/10.3390/molecules29020398
APA StyleZafar, A., Takeda, C., Manzoor, A., Tanaka, D., Kobayashi, M., Wadayama, Y., Nakane, D., Majeed, A., Iqbal, M. A., & Akitsu, T. (2024). Towards Industrially Important Applications of Enhanced Organic Reactions by Microfluidic Systems. Molecules, 29(2), 398. https://doi.org/10.3390/molecules29020398