Glassy Powder Derived from Waste Printed Circuit Boards for Methylene Blue Adsorption
Abstract
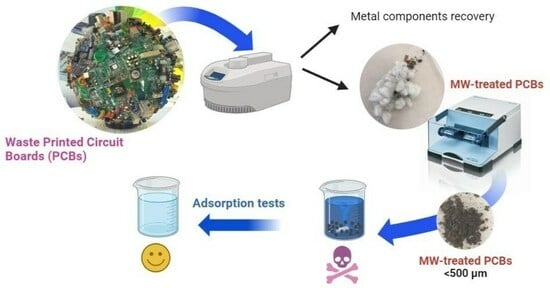
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. MW-Treated WPCB Samples’ Preparation and Characterization
3.2. Reference Adsorbents Preparation and Characterization
3.3. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Tan, Q.; Yu, J.; Wang, M. A global perspective on e-waste recycling. Circ. Econ. 2023, 2, 100028. [Google Scholar] [CrossRef]
- Zanoletti, A.; Cornelio, A.; Bontempi, E. A post-pandemic sustainable scenario: What actions can be pursued to increase the raw materials availability? Environ. Res. 2021, 202, 111681. [Google Scholar] [CrossRef] [PubMed]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-waste Monitor 2020: Quantities, Flows, and the Circular Economy Potential; United Nations University/United Nations Institute for Training and Research, International Telecommunication Union, and International Solid Waste Association: Bonn, Germany; Geneva, Switerland; Rotterdam, The Netherlands, 2020. [Google Scholar]
- Research and Markets. Electronics Recycling—Global Strategic Business Report; Research and Markets: Dublin, Ireland, 2023. [Google Scholar]
- Mir, S.; Dhawan, N. A comprehensive review on the recycling of discarded printed circuit boards for resource recovery. Resour. Conserv. Recycl. 2022, 178, 106027. [Google Scholar] [CrossRef]
- Niu, B.; Shanshan, E.; Xu, Z.; Guo, J. How to efficient and high-value recycling of electronic components mounted on waste printed circuit boards: Recent progress, challenge, and future perspectives. J. Clean. Prod. 2023, 415, 137815. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z. Disposing and recycling waste printed circuit boards: Disconnecting, resource recovery, and pollution control. Environ. Sci. Technol. 2015, 49, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Tembhare, S.P.; Bhanvase, B.A.; Barai, D.P.; Dhoble, S.J. E-waste recycling practices: A review on environmental concerns, remediation and technological developments with a focus on printed circuit boards. Environ. Dev. Sustain. 2022, 24, 8965–9047. [Google Scholar] [CrossRef]
- Grigorescu, R.M.; Grigore, M.E.; Iancu, L.; Ghioca, P.; Ion, R.M. Waste electrical and electronic equipment: A review on the identification methods for polymeric materials. Recycling 2019, 4, 32. [Google Scholar] [CrossRef]
- Elvinger, J.; Preiss, I.; Törneling, C.; Markus, R.; Roessing, E.; Coelho, J.; Williams, A.; Meisenzahl, M. EU Actions and Existing Challenges on E-Waste Review; European Court of Auditors: Luxembourg, 2021. [Google Scholar]
- Do Nascimento, J.D.R.V.; Wohnrath, K.; Garcia, J.R. Synthesis of gold nanoparticles using recovered gold from electronic waste. Orbital Electron. J. Chem. 2021, 13, 153–159. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Z. Recycling non-leaching gold from gold-plated memory cards: Parameters optimization, experimental verification, and mechanism analysis. J. Clean. Prod. 2017, 162, 1518–1526. [Google Scholar] [CrossRef]
- Luda, M.P. Recycling of Printed Circuit Boards. In Integrated Waste Management—Volume II; Kumar, S., Ed.; InTech: London, UK, 2011; pp. 285–298. ISBN 978-953-307-447-4. [Google Scholar]
- Ulman, K.; Ghose, A.; Maroufi, S.; Mansuri, I.; Sahajwalla, V. Disentanglement of random access memory cards to regenerate copper foil: A novel thermo-electrical approach. Waste Manag. 2018, 81, 138–147. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J.; Xu, Z. Pyrolysis-Based Technology for Recovering Copper from Transistors on Waste Printed Circuit Boards. ACS Sustain. Chem. Eng. 2017, 5, 11354–11361. [Google Scholar] [CrossRef]
- Rigoldi, A.; Trogu, E.F.; Marcheselli, G.C.; Artizzu, F.; Picone, N.; Colledani, M.; Deplano, P.; Serpe, A. Advances in Recovering Noble Metals from Waste Printed Circuit Boards (WPCBs). ACS Sustain. Chem. Eng. 2019, 7, 1308–1317. [Google Scholar] [CrossRef]
- Barragan, J.A.; Ponce De León, C.; Alemán Castro, J.R.; Peregrina-Lucano, A.; Gómez-Zamudio, F.; Larios-Durán, E.R. Copper and Antimony Recovery from Electronic Waste by Hydrometallurgical and Electrochemical Techniques. ACS Omega 2020, 5, 12355–12363. [Google Scholar] [CrossRef]
- Hadi, P.; Xu, M.; Lin, C.S.K.; Hui, C.W.; McKay, G. Waste printed circuit board recycling techniques and product utilization. J. Hazard. Mater. 2015, 283, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Reghunadhan, A.; Jibin, K.P.; Kaliyathan, A.V.; Velayudhan, P.; Strankowski, M.; Thomas, S. Shape memory materials from rubbers. Materials 2021, 14, 7216. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Mezgebe, B.; Dietrich, J.; Shan, Y.; Harmon, S.; Lee, C.C. Material recovery from electronic waste using pyrolysis: Emissions measurements and risk assessment. J. Environ. Chem. Eng. 2021, 9, 104943. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, X.; Ge, X.; Chen, M. Chemical pyrolysis of E-waste plastics: Char characterization. J. Environ. Manag. 2018, 214, 94–103. [Google Scholar] [CrossRef]
- Liou, T.H.; Jheng, J.Y. Synthesis of High-Quality Ordered Mesoporous Carbons Using a Sustainable Way from Recycling of E-waste as a Silica Template Source. ACS Sustain. Chem. Eng. 2018, 6, 6507–6517. [Google Scholar] [CrossRef]
- Zheng, Y.; Shen, Z.; Cai, C.; Ma, S.; Xing, Y. The reuse of nonmetals recycled from waste printed circuit boards as reinforcing fillers in the polypropylene composites. J. Hazard. Mater. 2009, 163, 600–606. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, B.; Yu, S.; Xiong, J.; Yao, Z.; Hu, B.; Yan, J. Waste-Printed Circuit Board Recycling: Focusing on Preparing Polymer Composites and Geopolymers. ACS Omega 2020, 5, 17850–17856. [Google Scholar] [CrossRef]
- Zheng, Y.; Shen, Z.; Ma, S.; Cai, C.; Zhao, X.; Xing, Y. A novel approach to recycling of glass fibers from nonmetal materials of waste printed circuit boards. J. Hazard. Mater. 2009, 170, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; Barford, J.; McKay, G. Toxic heavy metal capture using a novel electronic waste-based material—Mechanism, modeling and comparison. Environ. Sci. Technol. 2013, 47, 8248–8255. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; Barford, J.; McKay, G. Selective toxic metal uptake using an e-waste-based novel sorbent-Single, binary and ternary systems. J. Environ. Chem. Eng. 2014, 2, 332–339. [Google Scholar] [CrossRef]
- Hadi, P.; Gao, P.; Barford, J.P.; McKay, G. Novel application of the nonmetallic fraction of the recycled printed circuit boards as a toxic heavy metal adsorbent. J. Hazard. Mater. 2013, 252–253, 166–170. [Google Scholar] [CrossRef]
- Xu, M.; Hadi, P.; Chen, G.; McKay, G. Removal of cadmium ions from wastewater using innovative electronic waste-derived material. J. Hazard. Mater. 2014, 273, 118–123. [Google Scholar] [CrossRef]
- Mariyam, S.; Zuhara, S.; Al-Ansari, T.; Mackey, H.; McKay, G. Novel high capacity model for copper binary ion exchange on e-waste derived adsorbent resin. Adsorption 2022, 28, 185–196. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Vassalini, I.; Ribaudo, G.; Gianoncelli, A.; Casula, M.F.; Alessandri, I. Plasmonic hydrogels for capture, detection and removal of organic pollutants. Environ. Sci. Nano 2020, 7, 3888–3900. [Google Scholar] [CrossRef]
- Vassalini, I.; Gjipalaj, J.; Crespi, S.; Gianoncelli, A.; Mella, M.; Ferroni, M.; Alessandri, I. Alginate-Derived Active Blend Enhances Adsorption and Photocatalytic Removal of Organic Pollutants in Water. Adv. Sustain. Syst. 2020, 4, 1900112. [Google Scholar] [CrossRef]
- Vassalini, I.; Maddaloni, M.; Depedro, M.; De Villi, A.; Ferroni, M.; Alessandri, I. From Water for Water: PEDOT: PSS-Chitosan Beads for Sustainable Dyes Adsorption. Gels 2024, 10, 37. [Google Scholar] [CrossRef]
- Zanoletti, A.; Vassura, I.; Venturini, E.; Monai, M.; Montini, T.; Federici, S.; Zacco, A.; Treccani, L.; Bontempi, E. A new porous hybrid material derived from silica fume and alginate for sustainable pollutants reduction. Front. Chem. 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Dutta, B.K. On the adsorption and diffusion of Methylene Blue in glass fibers. J. Colloid Interface Sci. 2005, 286, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. VIII. Update. Adv. Colloid Interface Sci. 2020, 275, 102064. [Google Scholar] [CrossRef]
- Tsai, C.K.; Horng, J.J. Transformation of glass fiber waste into mesoporous zeolite-like nanomaterials with efficient adsorption of methylene blue. Sustainability 2021, 13, 6207. [Google Scholar] [CrossRef]
- Girgis, B.S.; Temerk, Y.M.; Gadelrab, M.M.; Abdullah, I.D. X-ray Diffraction Patterns of Activated Carbons Prepared under Various Conditions. Carbon Sci. 2007, 8, 95–100. [Google Scholar] [CrossRef]
- Shimodaira, N.; Masui, A. Raman spectroscopic investigations of activated carbon materials. J. Appl. Phys. 2002, 92, 902–909. [Google Scholar] [CrossRef]
- Chike, K.E.; Myrick, M.L.; Lyon, R.E.; Angel, S.M. Raman and near-infrared studies of an epoxy resin. Appl. Spectrosc. 1993, 47, 1631–1635. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javaid, S.; Zanoletti, A.; Serpe, A.; Bontempi, E.; Alessandri, I.; Vassalini, I. Glassy Powder Derived from Waste Printed Circuit Boards for Methylene Blue Adsorption. Molecules 2024, 29, 400. https://doi.org/10.3390/molecules29020400
Javaid S, Zanoletti A, Serpe A, Bontempi E, Alessandri I, Vassalini I. Glassy Powder Derived from Waste Printed Circuit Boards for Methylene Blue Adsorption. Molecules. 2024; 29(2):400. https://doi.org/10.3390/molecules29020400
Chicago/Turabian StyleJavaid, Saad, Alessandra Zanoletti, Angela Serpe, Elza Bontempi, Ivano Alessandri, and Irene Vassalini. 2024. "Glassy Powder Derived from Waste Printed Circuit Boards for Methylene Blue Adsorption" Molecules 29, no. 2: 400. https://doi.org/10.3390/molecules29020400
APA StyleJavaid, S., Zanoletti, A., Serpe, A., Bontempi, E., Alessandri, I., & Vassalini, I. (2024). Glassy Powder Derived from Waste Printed Circuit Boards for Methylene Blue Adsorption. Molecules, 29(2), 400. https://doi.org/10.3390/molecules29020400