Celastrol Pyrazine Derivative Alleviates Silicosis Progression via Inducing ROS-Mediated Apoptosis in Activated Fibroblasts
Abstract
:1. Introduction
2. Results
2.1. CEL-07 Inhibits Pulmonary Pathological Damage in CS-Induced Silicosis Mice
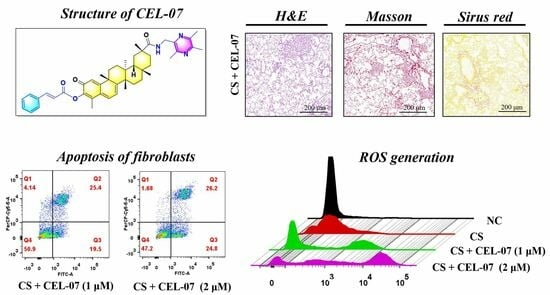
2.2. CEL-07 Suppresses CS-Induced Lung Fibrosis
2.3. CEL-07 Reduces Excessive Extracellular Matrix Deposition in Silicosis Mice
2.4. CEL-07 Improves Lung Respiratory Function of Silicosis Mice
2.5. CEL-07 Suppresses the Expression of Inflammatory and Fibrotic Factors in Silicosis Mice
2.6. CEL-07 Inhibits the Expression of Inflammatory and Fibrotic Factors In Vitro
2.7. CEL-07 Promotes CS-Induced Fibroblast Apoptosis
2.8. CEL-07 Promotes Fibroblast Apoptosis by Increasing ROS Generation
2.9. Bioinformatics Analysis of the Pathogenesis and Potential Pathways of Silicosis
2.10. Pathway Analysis of CEL-07 in Improving Silicosis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mouse Experiment
4.3. Differential Gene Expression in Pulmonary Fibrosis
4.4. Interaction Network Construction
4.5. Lung Function Assay (LFA)
4.6. Histological Staining and Analysis (HSA)
4.7. Cell Culture
4.8. Cell Viability Assay
4.9. ROS Generation Assay
4.10. Real-Time Quantitative PCR (RT-qPCR) Analysis
4.11. Analysis of Cancer Cell Apoptosis
4.12. Western Blot Assay
4.13. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Zhang, Z.; Liu, J.; Song, M.; Zhang, T.; Chen, Y.; Hu, H.; Yang, P.; Li, B.; Song, X.; et al. Gefitinib and fostamatinib target EGFR and SYK to attenuate silicosis: A multi-omics study with drug exploration. Signal Transduct. Target. Ther. 2022, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Căluțu, I.-M.; Smărăndescu, R.-A.; Rașcu, A. Biomonitoring Exposure and Early Diagnosis in Silicosis: A Comprehensive Review of the Current Literature. Biomedicines 2022, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Krefft, S.; Wolff, J.; Rose, C. Silicosis: An Update and Guide for Clinicians. Clin. Chest Med. 2020, 41, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Chen, S. The Mechanism and Effect of Autophagy, Apoptosis, and Pyroptosis on the Progression of Silicosis. Int. J. Mol. Sci. 2021, 22, 8110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sui, J.-N.; Gao, L.; Guo, J. Subcutaneous administration of infliximab-attenuated silica-induced lung fibrosis. Int. J. Occup. Med. Environ. Health 2018, 31, 503–515. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Mamoulaki, M.; Malagari, K.; Kritikos, H.D.; Bouros, D.; Siafakas, N.M.; Boumpas, D.T. Infliximab therapy in pulmonary fibrosis associated with collagen vascular disease. Clin. Exp. Rheumatol. 2007, 25, 23–28. [Google Scholar] [PubMed]
- He, X.; Chen, S.; Li, C.; Ban, J.; Wei, Y.; He, Y.; Liu, F.; Chen, Y.; Chen, J. Trehalose Alleviates Crystalline Silica-Induced Pulmonary Fibrosis via Activation of the TFEB-Mediated Autophagy-Lysosomal System in Alveolar Macrophages. Cells 2020, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Zhang, S.; Li, J.; Fang, H. Effects of tetrandrine combined with acetylcysteine on exercise tolerance, pulmonary function and serum TNF-β1 and MMP-7 in silicosis patients. Exp. Ther. Med. 2020, 19, 2195–2201. [Google Scholar] [CrossRef]
- Tan, S.; Yang, S.; Kang, H.; Zhou, K.; Wang, H.; Zhang, Y.; Chen, S. Atractylenolide III Ameliorated Autophagy Dysfunction via Epidermal Growth Factor Receptor-Mammalian Target of Rapamycin Signals and Alleviated Silicosis Fibrosis in Mice. Lab. Investig. 2023, 103, 100024. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.; Jia, Q.; Bo, C.; Shao, H.; Zhang, Z. Pirfenidone inhibits epithelial-mesenchymal transition and pulmonary fibrosis in the rat silicosis model. Toxicol. Lett. 2019, 300, 59–66. [Google Scholar] [CrossRef]
- Shochet, G.E.; Wollin, L.; Shitrit, D. Fibroblast–matrix interplay: Nintedanib and pirfenidone modulate the effect of IPF fibroblast-conditioned matrix on normal fibroblast phenotype. Respirology 2018, 23, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-M.; Cui, J.-Z.; Cui, Y.; Li, R.; Tian, Y.-X.; Song, S.-X.; Zhang, J.; Gao, J.-L. Therapeutic effect of exogenous bone marrow-derived mesenchymal stem cell transplantation on silicosis via paracrine mechanisms in rats. Mol. Med. Rep. 2013, 8, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, H.; Chen, M.; Wang, S.; Qian, R.; Zhang, L.; Huang, X.; Wang, J.; Liu, Z.; Qin, W.; et al. A survey of optimal strategy for signature-based drug repositioning and an application to liver cancer. Elife 2022, 11, e71880. [Google Scholar] [CrossRef]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, B.; Cloyd, J.M.; Alaimo, L.; Xu, G.; Du, S.; Mao, Y.; Pawlik, T.M. Novel Drug Candidate Prediction for Intrahepatic Cholangiocarcinoma via Hub Gene Network Analysis and Connectivity Mapping. Cancers 2022, 14, 3284. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Feng, Y.; He, W.; Xu, W.; Xu, W.; Yang, H.; Li, X. Celastrol in metabolic diseases: Progress and application prospects. Pharmacol. Res. 2021, 167, 105572. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Q.; Luo, P.; Gu, L.; Shen, S.; Tang, H.; Zhang, Y.; Lyu, M.; Shi, Q.; Yang, C.; et al. Neuroprotective Effects of Celastrol in Neurodegenerative Diseases-Unscramble Its Major Mechanisms of Action and Targets. Aging Dis. 2022, 13, 815–836. [Google Scholar] [CrossRef]
- Yu, X.; Meng, X.; Xu, M.; Zhang, X.; Zhang, Y.; Ding, G.; Huang, S.; Zhang, A.; Jia, Z. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-kappaB and improving mitochondrial function. EBioMedicine 2018, 36, 266–280. [Google Scholar] [CrossRef]
- Ye, S.; Luo, W.; Khan, Z.A.; Wu, G.; Xuan, L.; Shan, P.; Lin, K.; Chen, T.; Wang, J.; Hu, X.; et al. Celastrol Attenuates Angiotensin II–Induced Cardiac Remodeling by Targeting STAT3. Circ. Res. 2020, 126, 1007–1023. [Google Scholar] [CrossRef]
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, J.; Hernandez, M.A.S.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Biondini, D.; Balestro, E.; Sverzellati, N.; Cocconcelli, E.; Bernardinello, N.; Ryerson, C.J.; Spagnolo, P. Acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF): An overview of current and future therapeutic strategies. Expert Rev. Respir. Med. 2020, 14, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Luo, W.; Chen, S.; Lin, F.; Zhang, X.; Fan, S.; Shen, X.; Wang, Y.; Liang, G. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics 2020, 10, 10290–10308. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, H.; Ding, C.; Jiang, D.; Zhao, Z.; Li, Y.; Ding, X.; Gao, J.; Zhou, H.; Luo, C.; et al. Celastrol suppresses colorectal cancer via covalent targeting peroxiredoxin 1. Signal Transduct. Target. Ther. 2023, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Liu, B.; Xu, H. Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur. J. Med. Chem. 2020, 189, 112081. [Google Scholar] [CrossRef] [PubMed]
- Cascão, R.; Vidal, B.; Finnilä, M.A.J.; Lopes, I.P.; Teixeira, R.L.; Saarakkala, S.; Moita, L.F.; Fonseca, J.E. Effect of celastrol on bone structure and mechanics in arthritic rats. RMD Open 2017, 3, e000438. [Google Scholar] [CrossRef]
- Bai, Y.; Liang, C.; Zhou, J.; Liu, Y.; Wang, F.; Gao, J.; Wu, J.; Hu, D. Development of novel celastrol-ligustrazine hybrids as potent peroxiredoxin 1 inhibitors against lung cancer. Eur. J. Med. Chem. 2023, 259, 115656. [Google Scholar] [CrossRef]
- Liang, C.; Bai, Y.; Miao, R.; Yang, X.; Gao, L.; Liu, Y.; Zhou, J.; Guo, J.; Hu, D.; Wu, J. Celastrol as a candidate drug for silicosis: From bioinformatics and network pharmacology to experimental validation. Int. Immunopharmacol. 2023, 125, 111068. [Google Scholar] [CrossRef]
- Cooley, J.C.; Javkhlan, N.; Wilson, J.A.; Foster, D.G.; Edelman, B.L.; Ortiz, L.A.; Schwartz, D.A.; Riches, D.W.; Redente, E.F. Inhibition of antiapoptotic BCL-2 proteins with ABT-263 induces fibroblast apoptosis, reversing persistent pulmonary fibrosis. J. Clin. Investig. 2023, 8, 163762. [Google Scholar] [CrossRef]
- Diwanji, N.; Bergmann, A. An unexpected friend—ROS in apoptosis-induced compensatory proliferation: Implications for regeneration and cancer. Semin. Cell Dev. Biol. 2018, 80, 74–82. [Google Scholar] [CrossRef]
- Wang, J.; Hu, K.; Cai, X.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm. Sin. B 2022, 12, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ning, K.; Sun, M.L.; Zhang, X.A. Regulation and therapy, the role of JAK2/STAT3 signaling pathway in OA: A systematic review. Cell Commun. Signal 2023, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.-Y.; Koh, M.-S.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, C.; Wang, X.; Sun, X.; Zhang, R.; Chen, C.; Yu, M.; Liu, Y.; Zhu, Y.; Chen, J. Oridonin attenuates lung inflammation and fibrosis in silicosis via covalent targeting iNOS. Biomed. Pharmacother. 2022, 153, 113532. [Google Scholar] [CrossRef] [PubMed]
- Hoy, R.F.; Chambers, D.C. Silica-related diseases in the modern world. Allergy 2020, 75, 2785–2797. [Google Scholar] [CrossRef]
- Tan, S.; Chen, S. Macrophage Autophagy and Silicosis: Current Perspective and Latest Insights. Int. J. Mol. Sci. 2021, 22, 453. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Gu, J.; Chen, C.; Duanmu, J.; Miao, J.; Yao, W.; Tao, J.; Tu, M.; Xiong, B.; et al. Celastrol exerts anti-inflammatory effect in liver fibrosis via activation of AMPK-SIRT3 signalling. J. Cell. Mol. Med. 2020, 24, 941–953. [Google Scholar] [CrossRef]
- Tang, M.; Cao, X.; Zhang, K.; Li, Y.; Zheng, Q.-Y.; Li, G.-Q.; He, Q.-H.; Li, S.-J.; Xu, G.-L.; Zhang, K.-Q. Celastrol alleviates renal fibrosis by upregulating cannabinoid receptor 2 expression. Cell Death Dis. 2018, 9, 601. [Google Scholar] [CrossRef]
- De Wolde, S.D.; Hulskes, R.H.; Weenink, R.P.; Hollmann, M.W.; Van Hulst, R.A. The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis. Biomolecules 2021, 11, 1210. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Curro, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Huang, I.-H.; Chung, W.-H.; Wu, P.-C.; Chen, C.-B. JAK–STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R.; Ashrafi, G.H.; Akhavan-Niaki, H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Cao, L.; Massey, I.Y. Role of PI3K/Akt signaling pathway in cardiac fibrosis. Mol. Cell. Biochem. 2021, 476, 4045–4059. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Parrish, A.R.; Hill, M.A.; Meininger, G.A. N-cadherin, a vascular smooth muscle cell-cell adhesion molecule: Function and signaling for vasomotor control. Microcirculation 2014, 21, 208–218. [Google Scholar] [CrossRef]
- Qvarnström, O.F.; Simonsson, M.; Eriksson, V.; Turesson, I.; Carlsson, J. γH2AX and cleaved PARP-1 as apoptotic markers in irradiated breast cancer BT474 cellular spheroids. Int. J. Oncol. 2009, 35, 41–47. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, Y.; Lang, X.; Zhang, Y. Role of mitochondrial metabolic disorder and immune infiltration in diabetic cardiomyopathy: New insights from bioinformatics analysis. J. Transl. Med. 2023, 21, 66. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]











| Gene | Sequence 5′----3′ | |
|---|---|---|
| Human-β-actin | Foward | GCACAGAGCCTCGCCTT |
| Reverse | CCTTGCACATGCCGGAG | |
| Human-TNF-α | Foward | GAGGCCAAGCCCTGGTATG |
| Reverse | CGGGCCGATTGATCTCAGC | |
| Human-TGF-β | Foward | GCTGTATTGCAGACTTAGGACTG |
| Reverse | TTTTTGTTCCCACTCTGTGGTT | |
| Human-IL6 | Foward | CCCCAATTTCCAATGCTCTCC |
| Reverse | CGCACTAGGTTTGCCGAGTA | |
| Human-IL-1α | Foward | AGATGCCTGAGATACCCAAAACC |
| Reverse | CCAAGCACACCCAGTAGTCT | |
| Human-IL-1β | Foward | ATGATGGCTTATTACAGTGGCAA |
| Reverse | GTCGGAGATTCGTAGCTGGA | |
| Mouse-β-actin | Foward | AGGTCGGTGTGAACGGATTTG |
| Reverse | TGTAGACCATGTAGTTGAGGTCA- | |
| Mouse-TNF-α | Foward | CCCTCACACTCAGATCATCTTCT |
| Reverse | GCTACGACGTGGGCTACAG | |
| Mouse-TGF-β | Foward | CTCCCGTGGCTTCTAGTGC |
| Reverse | GCCTTAGTTTGGACAGGATCTG | |
| Mouse-IL-1α | Foward | GCAACTGTTCCTGAACTCAACT |
| Reverse | ATCTTTTGGGGTCCGTCAACT | |
| Mouse-IL-1β | Foward | CGAAGACTACAGTTCTGCCATT |
| Reverse | GACGTTTCAGAGGTTCTCAGAG | |
| Mouse-IL-6 | Foward | TAGTCCTTCCTACCCCAATTTCC |
| Reverse | TTGGTCCTTAGCCACTCCTTC | |
| Mouse-COL1A1 | Foward | ACGGCTGCACGAGTCACAC |
| Reverse | GGCAGGCGGGAGGTCTT | |
| Mouse-α-SMA | Foward | ACACGGCATCATCACCAACTG |
| Reverse | TCCAGAGTCCAGCACAATACCA | |
| Mouse-COL3A1 | Foward | CTGTAACATGGAAACTGGGGAAA CCATAGCTGAACTGAAAACCACC |
| Reverse |
| Antibody Name | Item No | Antibody Name | Item No |
|---|---|---|---|
| HRP Goat Anti-Rabbit IgG (H + L) | AS014, Abclonal | Phospho-c-Raf (Ser259) | #9421, CST |
| Phospho-Jak2 (Tyr1007/1008) | #3771, CST | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | #9101, CST |
| Phospho-Stat3 (Tyr705) | #9145, CST | N-Cadherin (D4R1H) | #13116, CST |
| Phospho-PI3 Kinase p85 (Tyr458) | #4228, CST | E-Cadherin (24E10) | #3195, CST |
| Phospho-Akt (Ser473) | #4060, CST | PARP (46D11) | #9532, CST |
| Cleaved PARP (Asp214) | #9541, CST | Anti-alpha smooth muscle Actin | #14968, CST |
| Anti-collagen I Rabbit pAb | GB114197-100, Servicebio | Anti- GAPDH antibody | GB15004-100, Servicebio |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Liang, C.; Gao, L.; Han, T.; Wang, F.; Liu, Y.; Zhou, J.; Guo, J.; Wu, J.; Hu, D. Celastrol Pyrazine Derivative Alleviates Silicosis Progression via Inducing ROS-Mediated Apoptosis in Activated Fibroblasts. Molecules 2024, 29, 538. https://doi.org/10.3390/molecules29020538
Bai Y, Liang C, Gao L, Han T, Wang F, Liu Y, Zhou J, Guo J, Wu J, Hu D. Celastrol Pyrazine Derivative Alleviates Silicosis Progression via Inducing ROS-Mediated Apoptosis in Activated Fibroblasts. Molecules. 2024; 29(2):538. https://doi.org/10.3390/molecules29020538
Chicago/Turabian StyleBai, Ying, Chao Liang, Lu Gao, Tao Han, Fengxuan Wang, Yafeng Liu, Jiawei Zhou, Jianqiang Guo, Jing Wu, and Dong Hu. 2024. "Celastrol Pyrazine Derivative Alleviates Silicosis Progression via Inducing ROS-Mediated Apoptosis in Activated Fibroblasts" Molecules 29, no. 2: 538. https://doi.org/10.3390/molecules29020538
APA StyleBai, Y., Liang, C., Gao, L., Han, T., Wang, F., Liu, Y., Zhou, J., Guo, J., Wu, J., & Hu, D. (2024). Celastrol Pyrazine Derivative Alleviates Silicosis Progression via Inducing ROS-Mediated Apoptosis in Activated Fibroblasts. Molecules, 29(2), 538. https://doi.org/10.3390/molecules29020538