1. Introduction
Food and green wastes constitute nearly half of the global waste generated, making them the largest waste category. It is projected that their volume will continue to rise, which is associated with technological and economic advancement, as well as population growth and consumption patterns [
1]. Among the primary waste products of the fruit industry is pomace, a by-product of juice extraction. The proportion of fruit pomace relative to the raw material utilized can range from 10% to 35%, depending on the processed fruit and the extraction technology employed [
2]. Inadequate management of these by-products could contribute to environmental risks and the release of hazardous greenhouse gases [
3,
4]. From an economic standpoint, such mismanagement is also inefficient as it results in the loss of valuable nutrients and health-promoting components, particularly in the context of malnutrition [
5]. It is estimated that only 20% of pomace finds useful applications, such as animal feed, organic fertilizer, or pectin extraction for ethanol production [
6,
7].
In recent years, there has been growing interest in utilizing fruit by-products in human nutrition, as they offer potential sources of ingredients such as fiber, phenolic compounds, vitamins, and minerals [
8]. Due to their high water content and the abundance of active compounds, prompt processing is necessary, especially for medium- and long-term storage. Moisture reduction techniques, such as drying, preserve the physical and chemical properties of the raw material, prevent spoilage, and reduce transportation costs [
9]. Incorporating fruit waste into food products may have a positive impact on public health by enhancing their nutritional value, and increasing fiber and polyphenolic intake [
10]. Furthermore, fortification can extend the shelf life of products and enhance their oxidative stability, while maintaining or even improving sensory properties [
8].
Pear pomace (PP), which includes the peel, pulp, stem, core, and seeds remaining after industrial juice production, is known to contain 44 to 79% fiber on a dry weight basis, as well as organic acids, triterpenes, and polyphenols [
11,
12]. Fresh PP can contain up to twice as many polyphenolic compounds as the whole fruit, with levels reaching up to 18 g·kg
−1 of fresh weight [
13]. Thus far, in PP, elevated concentrations of procyanidins have been documented, accompanied by the presence of hydroxycinnamic acids, particularly 5-caffeoylquinic acid. PP was also characterized by the presence of diverse organic acids, including malic acid, citric acid, and shikimic acid, alongside triterpenes such as oleanolic acid and ursolic acid. Furthermore, polymeric procyanidins and arbutin were identified as additional constituents in PP. Notably, anthocyanins, specifically cyanidin derivatives, exhibited exclusive localization within the peel of the fruit, while being absent in the pulp, seeds, and leaves [
14]. The aqueous extract of PP exhibited potential protective effects and the ability to inhibit lipid peroxidation, as observed in a study involving rats fed a high-fat/cholesterol diet [
12]. Moreover, PP demonstrated anti-obesity effects, as evidenced by its positive impact on glucose homeostasis and modulation of gut microbiota composition in mice [
15,
16]. The aforementioned studies strongly indicate that incorporating PP into food products as a means of enrichment is a promising and practical choice.
The drying conditions applied to fruit by-products significantly influence their grinding characteristics and quality, including factors such as color, activity, water content, chemical composition, and antioxidant properties [
17,
18]. However, there is a lack of information in the current literature regarding the effect of drying methods and temperatures specifically applied to PP on its grinding process and physicochemical properties, which, in turn, impact the food properties of the resulting product.
Therefore, this study aims to investigate the effects of contact-drying and freeze-drying at various hotplate temperatures on the drying kinetics, energy intensity of the pomace comminuting process, powder color, profile of phenolic compounds, as well as antioxidant activity.
3. Materials and Methods
3.1. Reagents and Raw Material
Gallic acid, methanol, ABTS (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid), DPPH (1,1-diphenyl-2-picrylhydrazyl), sodium bicarbonate, potassium persulfate, Folin and Ciocalteu′s phenol reagent, and standard phenolic compounds (
Table 9) of LC-MS/MS were analytical grade and were purchased from Sigma (Sigma-Aldrich GmbH, Steinheim, Germany).
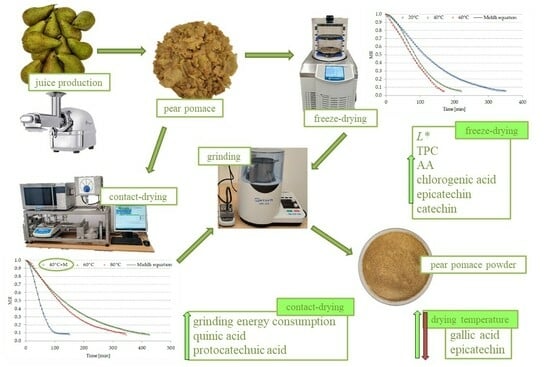
Full-ripe pears of the Konferencja variety were purchased from a local store and underwent a washing procedure. Subsequently, they were sliced, and their seed nests and stalks were removed. PP was extracted from the pears using a twin-screw juicer, Angel Juicer (Angel 5500, Angel Juicers, South Korea, Makpo) and then subjected to analysis. The part of raw material intended for freeze-drying was placed in a freezer chamber (GTL-4905, Liebherr, Sweden, Gothenburg) and frozen at −30 °C.
3.2. Drying Process
Freeze-drying (FD) and contact-drying (CD) experiments were conducted on PP. The FD process utilized an Alpha 1–4 Martin Christ freeze-dryer (Gefriertrocknungsanlagen GmbH in Osterode am Harz, Germany), employing a single-sided contact heat delivery method. Heating plates were subjected to three different temperatures (20 °C, 40 °C, and 60 °C with an accuracy of ± 2 °C) at a drying chamber pressure of 100 Pa. CD, more broadly referred to as air-drying, was conducted using a convection dryer (Promis-Tech, Wroclaw, Poland). The drying process involved placing a single layer of 100 g of the samples on trays exposed to an air velocity of 0.5 m/s (with an accuracy of ±0.1 m/s) at temperatures of 40 °C (with microwave assistance of 50 W), 60 °C, and 80 °C with an accuracy of ±1 °C). Microwaves were employed in CD, as a temperature of 40 °C did not yield a desiccated product suitable for proper pulverization. Contact-drying was chosen over convection drying due to the pomace’s pulp form, making it impractical to position the raw material for direct exposure to the drying agent. The FD and CD (100 g samples) procedures continued until the moisture content of the PP reached between 6 and 8%. During measurements taken every 5 min, changes in the mass of the dried material sample were constantly recorded.
3.3. Modeling of Drying Curves
The kinetics of the process of drying was estimated using changes in the reduced water content (MR) as a basis:
where u
t stands for the water content during the drying process (kg H
2O·kg
−1 DW) and u
0 represents the initial water content (kg H
2O·kg
−1 DW). The equilibrium water content in this equation was disregarded because its value is insignificant in relation to u
0 and u
t. This kind of simplification is frequently employed and has little effect on the drying kinetics results [
51].
To identify the most suitable mathematical model for capturing the kinetics of sublimation and convection drying of pear pomace, seven equations commonly cited in the literature were analyzed, as presented in
Table 10.
3.4. Moisture Content and Water Activity
To assess the moisture content of PP, the gravimetric method was employed. Five gram samples were subjected to drying in a laboratory dryer at 105 °C until a constant weight was achieved. The water activity was measured using a water activity measurement device (LabMaster, Novasina, Swiss, Lachen).
3.5. Grinding Energy and Particle Size Analysis
PP was subjected to grinding for 60 s using a knife grinder (GRINDOMIX GM-200, Retsch, Germany, Haan, 1000 W, 10,000 rpm). For the measurement and computation of grinding energy, a computer system was integrated into the mills to monitor and assess the amount of energy expended during grinding [
59]. The energy utilized in grinding was measured using a digital multimeter (VC870, Voltcraft
®, Germany, Wollerau), connected to a computer running a program (VC870 Interface 4.2.6., Voltcraft
®, Germany, Wollerau) that recorded the data every 1 min.
The particle size distribution of PP powder was assessed through laser light scattering employing a laser particle size analyzer (Malvern Mastersizer 3000, Malvern Instruments Ltd., Worcestershire, UK). In laser diffraction measurement, a 5 g sample of fruit powder was automatically analyzed for particle size by passing a laser beam through it. The size dispersion index (Span) was determined using the following equation:
where d
10, d
50, and d
90 represent diameters below which 10, 50, and 90% of the sample particles are smaller, respectively [
60]. Each sample underwent three repetitions.
The grinding efficiency index was calculated as the ratio between the surface area of the PP powder and the grinding energy. The specific grinding energy was determined by dividing the grinding energy by the mass of the PP powder [
61].
3.6. Color Coordinates
The CIE
Lab* system was employed to determine the color coordinates of PP using a colorimeter (NR20XE, Shenzhen Threenh Technology Co., Shenzhen, China). The system’s lightness, denoted as L*, ranges from 0 (perfect black body) to 100 (perfect white body), with
a* coordinate signifying the shift from green (−a*) to red (a*), and b* coordinate representing the transition from blue (−b*) to yellow (b*) [
62]. Hue angle was also calculated according to the equation:
3.7. Antioxidant Capacity
3.7.1. Preparation of Extracts
In total, 1000 mg of pear pomace powder was extracted for 30 min in a 5 mL methanol (100%):water (1:1, v/v) mixture, with stirring using a rotator (Multi Bio RS-24, Biosan Sia, Latvia, Riga). Subsequently, the samples were centrifuged for 5 min at 5000 rpm (3070× g) (LC8 3500, Benchmark, Sayreville, NJ, USA). The supernatant was collected, and the extraction procedure was repeated two more times. The resulting extracts were combined and stored in darkness at 20 °C.
3.7.2. Total Phenolics Content (TPC)
TPC was determined using the modified Folin–Ciocalteu method [
63]. Specifically, 0.1 mL of the extract was mixed with 0.1 mL of distilled water and 0.4 mL of the diluted Folin reagent (with water 1:5,
v/
v). After 3 min, 2 mL of 7% sodium carbonate was added to the mixture, followed by vigorous shaking for 1 min. After incubating for 30 min in a dark environment, absorbance was measured at 760 nm. TPC was quantified in milligrams per gram of dry weight as gallic acid equivalents (y = 0.0039x + 0.0142, R
2 = 0.9941). The blank sample was prepared using a methanol:water (1:1,
v/
v) mixture instead of the extract.
3.7.3. DPPH and ABTS Methods
The antioxidant activity by the DPPH method was determined following the protocol by Lisiecka and Wójtowicz [
64]. To assess the antioxidant activity, 2.5 mL of a freshly prepared 0.2 mM/L solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) in methanol was mixed with 0.1 mL of the extract. After 30-min incubation in a dark environment, the decrease in absorbance induced by the sample was measured at 515 nm. The absorbance of the DPPH solution was 0.7 ± 0.05.
The antioxidant activity using the ABTS method was conducted in accordance with the modified protocol by Re et al. [
65]. ABTS was dissolved to achieve a concentration of 7 mM in water. It was then mixed with potassium persulfate to reach a final concentration of 2.45 mM, generating ABTS+ radicals. The mixture was left in the dark at room temperature for 12 h before use. The resulting solution was diluted with distilled water to obtain an absorbance of 0.7 ± 0.02 at a wavelength of 734 nm. Subsequently, 0.6 mL of the extract was added to 2.7 mL of the ABTS+ solution, and the absorbance was measured after 15 min.
Measurements of TPC and AA were performed using the Spectrophotometer Model 9423 (Alt, East Lyme, CT, USA). The blank samples comprised methanol:water (1:1, v/v) mixture in place of the extract.
The radical scavenging activity was determined according to the following formula [
66]:
where A
blank is the absorption of a blank sample, and A
sample is the absorption of a tested sample with DPPH/ABTS reagent. The percentage of DPPH and ABTS inhibition is presented in
Figures S1 and S2 (
Supplementary Material).
The values for half-maximal inhibitory concentration (EC
50) were determined by calculating the concentration of the PP extract at which 50% of the maximum inhibition was achieved, as indicated by the fitted models employing a dose-dependent mode of action [
67].
3.8. Quantitative Analysis of Phytochemicals by LC–MS/MS
3.8.1. Preparation of Extracts
One gram of powdered PP underwent extraction using 10 mL of methanol in an ultrasonic bath for a duration of 3 h at 40 °C with a frequency of 28 kHz. These extraction conditions were selected based on prior experimentation. Subsequently, the tubes containing the extracted material underwent centrifugation. The resulting upper layer was then filtered through a 0.2 µm string filter.
3.8.2. Test Solution for Mass Spectrometer and Chromatography Conditions
The study outlined the analytical methodology employed was elucidated by Yilmaz [
68]. A Shimadzu–Nexera ultrahigh performance liquid chromatograph (UHPLC) coupled with a tandem mass spectrometer (USA, Columbia) was employed for the quantitative analysis of 53 phytochemicals. The UHPLC system, comprising an autosampler (SIL-30AC), a column oven (CTO-10ASvp), binary pumps (LC-30AD), and a degasser (DGU-20A3R) was utilized. The optimization of chromatographic conditions aimed to achieve the optimal separation of the 53 phytochemicals and mitigate suppression effects. Various columns, such as Agilent Poroshell 120 EC-C18 (150 mm × 2.1 mm, 2.7 µm) and RP-C18 Inertsil ODS-4 (100 mm × 2.1 mm, 2 µm), along with diverse mobile phases (B) including acetonitrile and methanol, and different mobile phase additives (ammonium formate, formic acid, ammonium acetate, and acetic acid) were investigated. Additionally, different column temperatures ranging from 25 °C to 40 °C were examined. The optimal chromatographic separation was achieved using an Agilent Poroshell 120 EC-C18 column (150 mm × 2.1 mm, 2.7 µm) at 40 °C. Eluent A (water + 5 mM ammonium formate + 0.1% formic acid) and eluent B (methanol + 5 mM ammonium formate + 0.1% formic acid) were used to create an elution gradient with the following profiles: 20–100% B (0–25 min), 100% B (25–35 min), and 20% B (35–45 min). The injection volume and flow rate of the solvent were set at 0.5 mL/min and 5 L, respectively. The Shimadzu LCMS-8040 tandem mass spectrometer, equipped with electrospray ionization (ESI) source operating in both negative and positive ionization modes, was employed for mass spectrometric detection. The LC-ESI-MS/MS data were processed using LabSolutions software (version 5.97, Shimadzu). The MRM (multiple reaction monitoring) mode was optimized to selectively detect and quantify phytochemical compounds based on specified precursor-to-fragment ion transitions. Collision energies (CE) were optimized to achieve optimal phytochemical fragmentation and maximal transmission of desired product ions. The MS operating conditions included a drying gas (N2) flow of 15 L/min, nebulizing gas (N2) flow of 3 L/min, DL temperature at 250 °C, heat block temperature at 400 °C, and interface temperature at 350 °C.
Since the LC-MS/MS method utilized had been previously developed and validated, comprehensive information regarding the method’s validation and development can be found in the study by Yilmaz, 2020 [
68].
3.9. Statistical Analysis of Data
The analysis was conducted in triplicate, and the acquired test results underwent statistical analysis, involving the calculation of mean values and standard deviations. Subsequently, a one-way analysis of variance was performed, and the Tukey test was employed to assess the significance of differences between the means. The regression analysis was used to evaluate the drying kinetics of PP, and the coefficient of determination (R2), mean-square error (RMSE), and Chi-quadrate test (χ
2) were calculated [
69]. The statistical analysis was conducted utilizing Statistica 14.0 software (StatSoft, Inc., Tulsa, OK, USA), with a significance level of α = 0.05 considered as an indicator of statistical importance.
4. Conclusions
Contact-drying of PP at 40 °C assisted by microwaves proved to be the fastest among the selected drying methods. Additionally, freeze-drying exhibited a shorter duration compared to the contact-drying method at the same temperature of 60 °C. Lyophilized PP, being more susceptible to comminution, required less energy input for size reduction compared to contact-dried pomace. Furthermore, freeze-dried PP displayed a brighter, less yellow, and less red coloration than the contact-dried raw material.
The lyophilization of PP resulted in an increase in TPC and improved AA compared to contact-drying, whether or not microwave assistance was applied. The elevation of the contact-drying temperature and the use of microwave assistance during contact-drying positively influenced the TPC and AA of PP.
In terms of specific phenolic components, PP dried through contact-drying at 60 °C showed higher levels of quinic and protocatechuic acids compared to lyophilized pomace at the same temperature. Conversely, lyophilized pomace exhibited increased concentrations of chlorogenic acid, epicatechin, and catechin. The content of certain phenolic components of PP, such as gallic acid and epicatechin, varied depending on the applied drying temperature.
Taking into consideration both TPC and AA, the most favorable PP powder was obtained through freeze-drying at a temperature of 20 °C.