Evaluation of Environmental Factor Effects on the Polyphenol and Flavonoid Content in the Leaves of Chrysanthemum indicum L. and Its Habitat Suitability Prediction Mapping
Abstract
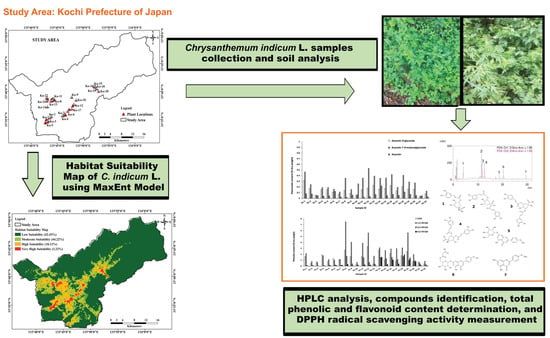
:1. Introduction
2. Results and Discussion
2.1. Soil Analysis
2.2. The Phytochemical Content and Antioxidant Activity
2.3. Habitat Suitability Map (HSM) of MaxEnt Model
2.4. Validation of MaxEnt Model
3. Materials and Methods
3.1. Study Area
3.2. Plant Materials and Data Collection
3.3. Chemicals
3.4. Instrumentation
3.5. Soil Sample Collection and Analysis
3.6. Preparation of Plant Extract and Samples
3.7. Evaluation of DPPH Radical Scavenging Activity
3.8. Total Phenolic Content
3.9. Total Flavonoid Content
3.10. Chromatographic Quantification and Analysis
3.11. MaxEnt Model
3.11.1. Dataset Preparation for Habitat Suitability Modeling
3.11.2. Validation of HSM
3.12. Statistical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadizadeh, H.; Samiei, L.; Shakeri, A. Chrysanthemum, an Ornamental Genus with Considerable Medicinal Value: A Comprehensive Review. S. Afr. J. Bot. 2022, 144, 23–43. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, D.; Yi, Y.-S.; Kim, J.H.; Jeong, H.Y.; Hwang, K.; Kim, J.-H.; Park, J.; Cho, J.Y. AKT-Targeted Anti-Inflammatory Activity of the Methanol Extract of Chrysanthemum indicum Var. Albescens. J. Ethnopharmacol. 2017, 201, 82–90. [Google Scholar] [CrossRef]
- Society of Japanese Pharmacopoeia. The Japanese Pharmacopoeia, 18th ed.; Pharmaceuticals and Medical Devices Agency: Tokyo, Japan, 2021. [Google Scholar]
- Gong, J.; Chu, B.; Gong, L.; Fang, Z.; Zhang, X.; Qiu, S.; Wang, J.; Xiang, Y.; Xiao, G.; Yuan, H.; et al. Comparison of Phenolic Compounds and the Antioxidant Activities of Fifteen Chrysanthemum morifolium Ramat Cv. ‘Hangbaiju’ in China. Antioxidants 2019, 8, 325. [Google Scholar] [CrossRef]
- Lee, D.Y.; Choi, G.; Yoon, T.; Cheon, M.S.; Choo, B.K.; Kim, H.K. Anti-Inflammatory Activity of Chrysanthemum indicum Extract in Acute and Chronic Cutaneous Inflammation. J. Ethnopharmacol. 2009, 123, 149–154. [Google Scholar] [CrossRef]
- Jeong, S.C.; Kim, S.M.; Jeong, Y.T.; Song, C.H. Hepatoprotective Effect of Water Extract from Chrysanthemum indicum L. Flower. Chin. Med. 2013, 8, 7. [Google Scholar] [CrossRef]
- Chen, M.; Wang, K.; Zhang, Y.; Zhang, M.; Ma, Y.; Sun, H.; Jin, Z.; Zheng, H.; Jiang, H.; Yu, P.; et al. New Insights into the Biological Activities of Chrysanthemum morifolium: Natural Flavonoids Alleviate Diabetes by Targeting α-Glucosidase and the PTP-1B Signaling Pathway. Eur. J. Med. Chem. 2019, 178, 108–115. [Google Scholar] [CrossRef]
- Ma, D.; Wako, Y. Evaluation of Phenolic Compounds and Neurotrophic/Neuroprotective Activity of Cultivar Extracts Derived from Chrysanthemum morifolium Flowers. Food Sci. Technol. Res. 2017, 23, 457–467. [Google Scholar] [CrossRef]
- Choi, K.T.; Kim, J.H.; Cho, H.T.; Lim, S.S.; Kwak, S.S.; Kim, Y.J. Dermatologic Evaluation of Cosmetic Formulations Containing Chrysanthemum indicum Extract. J. Cosmet. Dermatol. 2016, 15, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Sawa, T.; Murayama, K.; Yamaguchi, S.; Miyazaki, Y.; Tachibana, H. Histamine Release-Suppressive Effect of Water Extracts Prepared from Flower and Leaf of Chrysanthemum, Shiranui Himekiku. Nippon. Shokuhin Kagaku Kogaku Kaishi 2012, 59, 394–400. [Google Scholar] [CrossRef]
- Shim, S.-Y.; Kang, H.-S.; Sun, H.-J.; Lee, Y.-J.; Park, J.-R.; Chun, S.-S.; Song, Y.-H.; Byun, D.-S. Isolation and Identification of Flavonoids from Gujeolcho (Chrysanthemum zawadskii Var. latilobum) as Inhibitor of Histamine Release. Food Sci. Biotechnol. 2012, 21, 613–617. [Google Scholar] [CrossRef]
- Lai, J.-P.; Lim, Y.H.; Su, J.; Shen, H.-M.; Ong, C.N. Identification and Characterization of Major Flavonoids and Caffeoylquinic Acids in Three Compositae Plants by LC/DAD-APCI/MS. J. Chromatogr. B 2007, 848, 215–225. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; Jiang, Z.; Zafar, S.; Xie, Q.; Yang, Y.; Liu, Y.; Yuan, H.; Jian, Y.; Wang, W. Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus. Molecules 2021, 26, 3038. [Google Scholar] [CrossRef]
- Liu, C.-C.; Zhang, Y.; Dai, B.-L.; Ma, Y.-J.; Zhang, Q.; Wang, Y.; Yang, H. Chlorogenic Acid Prevents Inflammatory Responses in IL-1β-Stimulated Human SW-1353 Chondrocytes, a Model for Osteoarthritis. Mol. Med. Rep. 2017, 16, 1369–1375. [Google Scholar] [CrossRef]
- Hunyadi, A.; Martins, A.; Hsieh, T.-J.; Seres, A.; Zupkó, I. Chlorogenic Acid and Rutin Play a Major Role in the In Vivo Anti-Diabetic Activity of Morus alba Leaf Extract on Type II Diabetic Rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef]
- Hong, S.; Joo, T.; Jhoo, J.-W. Antioxidant and Anti-Inflammatory Activities of 3,5-Dicaffeoylquinic Acid Isolated from Ligularia fischeri Leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]
- Yao, H.; Shang, Z.; Wang, P.; Li, S.; Zhang, Q.; Tian, H.; Ren, D.; Han, X. Protection of Luteolin-7-O-Glucoside Against Doxorubicin-Induced Injury Through PTEN/Akt and ERK Pathway in H9c2 Cells. Cardiovasc. Toxicol. 2016, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cao, W.; Yao, J.; Yuan, Y.; Hong, Y.; Wang, X.; Xing, J. Cardioprotective Effects of Tilianin in Rat Myocardial Ischemia-Reperfusion Injury. Mol. Med. Rep. 2015, 11, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Milbradt, R. Skin Anti-Inflammatory Activity of Apigenin-7-Glucoside in Rats. Arzneimittelforschung 1993, 43, 370–372. [Google Scholar] [PubMed]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Marjanovic Vicentic, J.; Popovic, J.; Golic Grdadolnik, S.; Markovic, D.; Sankovic-Babice, S.; Glamoclija, J.; et al. Apigenin-7-O-Glucoside versus Apigenin: Insight into the Modes of Anticandidal and Cytotoxic Actions. EXCLI J. 2017, 16, 795–807. [Google Scholar] [CrossRef]
- Singh, R.P. Acacetin Inhibits Cell Growth and Cell Cycle Progression, and Induces Apoptosis in Human Prostate Cancer Cells: Structure-Activity Relationship with Linarin and Linarin Acetate. Carcinogenesis 2005, 26, 845–854. [Google Scholar] [CrossRef]
- Rajalakshmi, G.; Komathi, S.; Raviganesh, B.; Poongodi, N.; Sasikala, T. In-Vitro Micropropagation and Antimicrobial Activity of Chrysanthemum indicum. Sch. Acad. J. Pharm. 2013, 2, 285–288. [Google Scholar]
- Li, Y.; Liu, X.-J.; Su, S.-L.; Yan, H.; Guo, S.; Qian, D.-W.; Duan, J.-A. Evaluation of Anti-Inflammatory and Antioxidant Effects of Chrysanthemum Stem and Leaf Extract on Zebrafish Inflammatory Bowel Disease Model. Molecules 2022, 27, 2114. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Hwang, J.-W.; Park, P.-J.; Jeong, J.-H. Antioxidant Activity and Protective Effects of Extracts from Chrysanthemum boreale on t-BHP Induced Oxidative Stress in Chang Cells. J. Korean Soc. Food Sci. Nutr. 2014, 43, 60–66. [Google Scholar] [CrossRef]
- Wang, T.; Shen, X.-G.; Guo, Q.-S.; Zhou, J.-S.; Mao, P.-F.; Shen, Z.-G. Comparison of Major Bioactive Components from Leaves of Chrysanthemum morifolium. China J. Chin. Mater. Medica 2015, 40, 1670–1675. [Google Scholar] [CrossRef]
- Alsaadi, D.H.M.; Raju, A.; Kusakari, K.; Karahan, F.; Sekeroglu, N.; Watanabe, T. Phytochemical Analysis and Habitat Suitability Mapping of Glycyrrhiza glabra L. Collected in the Hatay Region of Turkey. Molecules 2020, 25, 5529. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The Effect of Water Stress on Phytochemical Accumulation, Bioactive Compounds and Expression of Key Genes Involved in Flavonoid Biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.-C. Chrysanthemum Morphology, Photosynthetic Efficiency and Antioxidant Capacity Are Differentially Modified by Light Quality. J. Plant Physiol. 2017, 213, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hirzel, A.; Guisan, A. Which Is the Optimal Sampling Strategy for Habitat Suitability Modelling. Ecol. Modell. 2002, 157, 331–341. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Convertino, M.; Muñoz-Carpena, R.; Chu-Agor, M.L.; Kiker, G.A.; Linkov, I. Untangling Drivers of Species Distributions: Global Sensitivity and Uncertainty Analyses of MaxEnt. Environ. Model. Softw. 2014, 51, 296–309. [Google Scholar] [CrossRef]
- Han, A.-R.; Nam, B.; Kim, B.-R.; Lee, K.-C.; Song, B.-S.; Kim, S.; Kim, J.-B.; Jin, C. Phytochemical Composition and Antioxidant Activities of Two Different Color Chrysanthemum Flower Teas. Molecules 2019, 24, 329. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Igarashi, K. Identification of Major Flavonoids in Petals of Edible Chrysanthemum Flowers and Their Suppressive Effect on Carbon Tetrachloride-Induced Liver Injury in Mice. Food Sci. Technol. Res. 2009, 15, 499–506. [Google Scholar] [CrossRef]
- Dey, S.K.; Mukherjee, A. Catechol Oxidase and Phenoxazinone Synthase: Biomimetic Functional Models and Mechanistic Studies. Coord. Chem. Rev. 2016, 310, 80–115. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.-H.; Zhang, X.-Y.; Zhang, L.-Y.; Zhao, P.-L.; Wen, T.; Zhang, J.-Q.; Xu, W.-L.; Guo, F.; Zhao, H.; et al. Exploring the Effects of Magnesium Deficiency on the Quality Constituents of Hydroponic-Cultivated Tea (Camellia sinensis L.) Leaves. J. Agric. Food Chem. 2021, 69, 14278–14286. [Google Scholar] [CrossRef] [PubMed]
- Manrique, L.A.; Jones, C.A.; Dyke, P.T. Predicting Cation-Exchange Capacity from Soil Physical and Chemical Properties. Soil. Sci. Soc. Am. J. 1991, 55, 787–794. [Google Scholar] [CrossRef]
- Cui, X.; Mao, P.; Sun, S.; Huang, R.; Fan, Y.; Li, Y.; Li, Y.; Zhuang, P.; Li, Z. Phytoremediation of Cadmium Contaminated Soils by Amaranthus hypochondriacus L.: The Effects of Soil Properties Highlighting Cation Exchange Capacity. Chemosphere 2021, 283, 131067. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the Environment on the Secondary Metabolic Profile of Tithonia diversifolia: A Model for Environmental Metabolomics of Plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. General Information on Climate of Japan. Available online: https://www.jma.go.jp/jma/indexe.html (accessed on 10 April 2021).
- Delta-T Devices Ltd. User Manual for the SM150T-UM-0.f. Soil Moisture Sensor; Delta-T Devices Ltd.: Cambridge, UK, 2016. [Google Scholar]
- Oki, T.; Masuda, M.; Furuta, S.; NishibaI, Y.; Suda, I. Radical Scavenging Activity of Fried Chips Made from Purple-Fleshed Sweet Potato. Nippon Shokuhin Kagaku Kogaku Kaishi 2001, 48, 926–932. [Google Scholar] [CrossRef]
- Zar Wynn Myint, K.; Kido, T.; Kusakari, K.; Prasad Devkota, H.; Kawahara, T.; Watanabe, T. Rhusflavanone and Mesuaferrone B: Tyrosinase and Elastase Inhibitory Biflavonoids Extracted from the Stamens of Mesua ferrea L. Nat. Prod. Res. 2021, 35, 1024–1028. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic Constituents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Zhou, C.; Meng, J.; Sun, J.; Zhou, T.; Tao, J. Impact of Climate Factors on Future Distributions of Paeonia ostii across China Estimated by MaxEnt. Ecol. Inform. 2019, 50, 62–67. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Blahut, J.; van Westen, C.J.; Sterlacchini, S. Analysis of Landslide Inventories for Accurate Prediction of Debris-Flow Source Areas. Geomorphology 2010, 119, 36–51. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Feng, Y.; Liu, Z. The Use of ROC and AUC in the Validation of Objective Image Fusion Evaluation Metrics. Signal Process. 2015, 115, 38–48. [Google Scholar] [CrossRef]









| ID a | Total Phenolic Content (mg Gallic Acid g−1 DW) b | Total Flavonoid Content (mg Quercetin g−1 DW) b | EC50 for DPPH Radical Scavenging (µg mL−1) c |
|---|---|---|---|
| Ko-1 | 27.2 ± 0.1 | 6.2 ± 0.1 | 69.8 ± 1.5 |
| Ko-2 | 17.5 ± 0.1 | 6.3 ± 0.6 | 92.2 ± 1.3 |
| Ko-3 | 15.0 ± 0.7 | 2.3 ± 0.0 | 123.2 ± 3.5 |
| Ko-4 | 28.3 ± 0.1 | 5.5 ± 0.0 | 49.0 ± 3.9 |
| Ko-5 | 18.9 ± 0.2 | 4.1 ± 0.1 | 61.3 ± 10.0 |
| Ko-6 | 22.7 ± 0.2 | 8.0 ± 0.0 | 74.2 ± 2.7 |
| Ko-7 | 23.6 ± 0.0 | 6.9 ± 0.0 | 60.8 ± 6.6 |
| Ko-8 | 64.1 ± 0.2 | 6.4 ± 0.1 | 29.4 ± 4.7 |
| Ko-9 | 38.6 ± 0.7 | 8.9 ± 0.1 | 39.8 ± 4.5 |
| Ko-10 | 15.6 ± 0.5 | 6.9 ± 0.2 | 65.9 ± 1.8 |
| Ko-11 | 48.6 ± 1.1 | 6.7 ± 0.0 | 31.9 ± 0.2 |
| Ko-12 | 61.9 ± 0.3 | 10.6 ± 0.1 | 32.7 ± 0.8 |
| Ko-13 | 21.9 ± 0.1 | 2.5 ± 0.2 | 57.7 ± 1.9 |
| Ko-14 | 55.8 ± 0.8 | 10.5 ± 0.0 | 34.1 ± 0.8 |
| Ko-15 | 40.1 ± 1.6 | 7.4 ± 0.1 | 38.0 ± 0.6 |
| Ko-16 | 53.0 ± 0.6 | 11.4 ± 0.1 | 32.2 ± 3.0 |
| Ko-17 | 41.5 ± 0.2 | 6.9 ± 0.1 | 28.0 ± 1.6 |
| Ko-18 | 56.4 ± 0.3 | 10.3 ± 0.1 | 30.7 ± 4.3 |
| Ko-19 | 53.1 ± 0.6 | 9.1 ± 0.0 | 36.2 ± 7.6 |
| Ko-20 | 30.0 ± 0.1 | 5.9 ± 0.1 | 38.3 ± 1.2 |
| Ko-21 | 17.5 ± 0.0 | 4.2 ± 0.0 | 76.0 ± 1.3 |
| Ko-22 | 15.2 ± 0.2 | 3.2 ± 0.0 | 76.5 ± 2.2 |
| Peak No. | Identified Compound | Observed Ion (Ion–Trap MS) | Retention Time (min.) |
|---|---|---|---|
| 1 | Chlorogenic acid | m/z [M + H]+: 355.0, m/z [M − H]−: 353.1 | 4.1 |
| 2 | 1,5-dicaffeoylquinic acid | m/z [M + H]+: 517.1, m/z [M − H]−: 515.2 | 10.7 |
| 3 | 3,5-dicaffeoylquinic acid | m/z [M − H]−: 515.1 | 11.1 |
| 4 | 4,5-dicaffeoylquinic acid | m/z [M + H]+: 517.1, m/z [M − H]−: 515.1 | 12.1 |
| 5 | Acacetin 7-O-glucoside | m/z [M + H]+: 447.0, m/z [M + H − 162]+: 285.0 | 18.4 |
| 6 | Acacetin 7-O-malonylglucoside | m/z [M + H]+: 533.1, m/z [M + H − 248]+: 285.0 | 20.0 |
| 7 | Acacetin | m/z [M + H]+: 285.0, m/z [M − H]−: 283.0 | 29.2 |
| Variables | CGA (%) | 1,5-DCQA (%) | 3,5-DCQA (%) | 4,5-DCQA (%) | Acacetin 7-O-Malonylglucoside (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F Value | R2 | F Value | R2 | F Value | R2 | F Value | R2 | F Value | R2 | |
| Exchangeable magnesium | 2.25 | 0.85 | 4.12 * | 0.91 | 17.87 *** | 0.98 | 90.23 *** | 0.99 | 1.21 | 0.75 |
| Cation exchangeable capacity | 3.61 * | 0.69 | 5.01 * | 0.76 | 2.98 * | 0.65 | 16.61 *** | 0.91 | 2.05 | 0.65 |
| BIO12 | 1.26 | 0.49 | 1.31 | 0.50 | 2.66 | 0.67 | 13.21 *** | 0.91 | 1.09 | 0.45 |
| pH | 0.70 | 0.48 | 1.79 | 0.7 | 11.48 *** | 0.94 | 2.07 | 0.73 | 4.73 * | 0.86 |
| Annual mean temperature and precipitation | 1.22 | 0.57 | 3.11 * | 0.77 | 5.34 * | 0.86 | 9.73 ** | 0.92 | 1.47 | 0.62 |
| BIO01 | 1 | 0.53 | 2.09 | 0.70 | 4.74 * | 0.84 | 9.65 ** | 0.91 | 0.88 | 0.49 |
| Clay | 0.66 | 0.17 | 1.13 | 0.26 | 2.91 | 0.48 | 9.09 *** | 0.74 | 1.13 | 0.26 |
| Bulk density | 2.21 | 0.88 | 4.25 | 0.93 | 8.70 * | 0.97 | 4.03 | 0.93 | 0.87 | 0.74 |
| TWI | 2.15 | 0.90 | 4.20 | 0.95 | 7.34 * | 0.97 | 3.04 | 0.93 | 4.54 | 0.95 |
| ID a | Longitude (E) | Latitude (N) | Elevation (m) | Mean Annual Temperature (°C) b | Mean Annual Rainfall (mm) b |
|---|---|---|---|---|---|
| Ko-1 | 133°43′50.4″ | 33°35′33.12″ | 326 | 15.9 | 2059 |
| Ko-2 | 133°43′35.5″ | 33°35′20.82″ | 296 | 16.3 | 2054 |
| Ko-3 | 133°43′14.59″ | 33°34′48.65″ | 197 | 16.5 | 2055 |
| Ko-4 | 133°43′14.34″ | 33°34′48.1″ | 199 | 16.5 | 2055 |
| Ko-5 | 133°42′58.3″ | 33°34′38.06″ | 226 | 16.5 | 2055 |
| Ko-6 | 133°42′59.08″ | 33°34′35.9″ | 220 | 16.5 | 2055 |
| Ko-7 | 133°42′57.57″ | 33°34′38.28″ | 232 | 16.5 | 2055 |
| Ko-8 | 133°44′6.93″ | 33°38′28.55″ | 141 | 15.7 | 2017 |
| Ko-9 | 133°43′55.26″ | 33°38′36.82″ | 191 | 15.3 | 2042 |
| Ko-10 | 133°43′59.79″ | 33°38′33.49″ | 214 | 15.2 | 2045 |
| Ko-11 | 133°44′21.54″ | 33°38′46.85″ | 301 | 15.2 | 2045 |
| Ko-12 | 133°44′11.05″ | 33°38′44.76″ | 322 | 15.2 | 2045 |
| Ko-13 | 133°43′49.03″ | 33°38′19.7″ | 86 | 16.1 | 1995 |
| Ko-14 | 133°43′6.83″ | 33°37′18.78″ | 176 | 16.6 | 2000 |
| Ko-15 | 133°52′20.23″ | 33°40′50.78″ | 275 | 14.3 | 2049 |
| Ko-16 | 133°52′23.51″ | 33°40′50.72″ | 263 | 14.3 | 2049 |
| Ko-17 | 133°52′18.18″ | 33°40′5″ | 273 | 14.3 | 2049 |
| Ko-18 | 133°52′36.35″ | 33°40′30.73″ | 292 | 13.9 | 2073 |
| Ko-19 | 133°52′36.33″ | 33°40′30.54″ | 293 | 13.9 | 2073 |
| Ko-20 | 133°52′59.64″ | 33°40′5.54″ | 296 | 14.0 | 2078 |
| Ko-21 | 133°44′24.09″ | 33°38′32.58″ | 97 | 15.2 | 2045 |
| Ko-22 | 133°43′8.01″ | 33°38′35.53″ | 75 | 15.6 | 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uranishi, R.; Aedla, R.; Alsaadi, D.H.M.; Wang, D.; Kusakari, K.; Osaki, H.; Sugimura, K.; Watanabe, T. Evaluation of Environmental Factor Effects on the Polyphenol and Flavonoid Content in the Leaves of Chrysanthemum indicum L. and Its Habitat Suitability Prediction Mapping. Molecules 2024, 29, 927. https://doi.org/10.3390/molecules29050927
Uranishi R, Aedla R, Alsaadi DHM, Wang D, Kusakari K, Osaki H, Sugimura K, Watanabe T. Evaluation of Environmental Factor Effects on the Polyphenol and Flavonoid Content in the Leaves of Chrysanthemum indicum L. and Its Habitat Suitability Prediction Mapping. Molecules. 2024; 29(5):927. https://doi.org/10.3390/molecules29050927
Chicago/Turabian StyleUranishi, Rei, Raju Aedla, Doaa H. M. Alsaadi, Dongxing Wang, Ken Kusakari, Hirotaka Osaki, Koji Sugimura, and Takashi Watanabe. 2024. "Evaluation of Environmental Factor Effects on the Polyphenol and Flavonoid Content in the Leaves of Chrysanthemum indicum L. and Its Habitat Suitability Prediction Mapping" Molecules 29, no. 5: 927. https://doi.org/10.3390/molecules29050927
APA StyleUranishi, R., Aedla, R., Alsaadi, D. H. M., Wang, D., Kusakari, K., Osaki, H., Sugimura, K., & Watanabe, T. (2024). Evaluation of Environmental Factor Effects on the Polyphenol and Flavonoid Content in the Leaves of Chrysanthemum indicum L. and Its Habitat Suitability Prediction Mapping. Molecules, 29(5), 927. https://doi.org/10.3390/molecules29050927