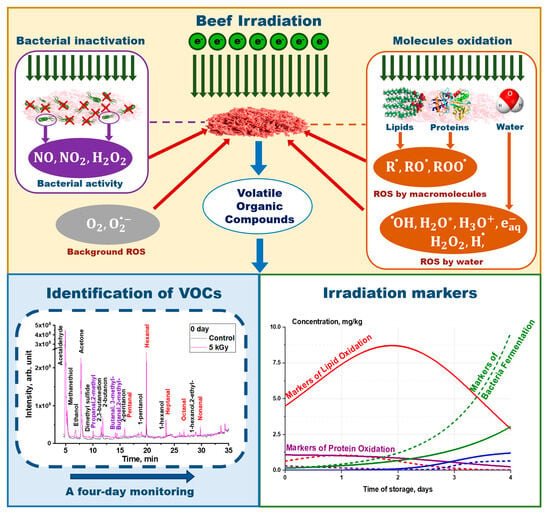
2.1. Volatile Organic Compounds Identified in Irradiation Beef Meat
The GC/MS analysis revealed the presence of 19 to 27 compounds in the beef samples, including alcohols, aldehydes, ketones, alkanes, and sulfur-containing compounds, depending on the exposure dose and storage time.
Figure 2 shows the detected classes of compounds, including the ratio between the summary concentrations of the compounds of different classes measured immediately after irradiation (
Figure 2A) and on day 2 (
Figure 2B) and day 4 (
Figure 2C) of observation in the non-irradiated beef samples and in the samples irradiated with a dose of 5 kGy. The radar chart below illustrates the kinetics of the change in VOC composition over time: red shows the composition of VOCs identified in the non-irradiated beef samples, and gray shows the composition of VOCs identified in the beef samples irradiated with 5 kGy.
The most common compounds identified in the beef samples during the observation are aldehydes and alcohols, which are also commonly found in other types of meat and poultry, such as pork [
27], lamb [
28], rabbit [
29], chicken [
30], turkey [
31], and duck [
32]. Immediately after irradiation, alcohols and sulfur-containing compounds that were not detected in the non-irradiated beef samples were recorded in the beef samples irradiated with different doses, and there is a certain dependence of concentration on the irradiation dose. At the beginning of the study, some compounds, later found in both non-irradiated and irradiated beef samples, were absent and appeared gradually over time (
Figure 2A). At the same time, some ketones were identified in the non-irradiated and irradiated beef samples within two days after irradiation (
Figure 2B) to disappear over the storage time. Interestingly, in the irradiated beef samples, all the aldehydes that were detected immediately after irradiation could also be found during all four days of observation (
Figure 2). Most of the alcohols, on the other hand, were detected after two days of storage in both non-irradiated and irradiated samples (
Figure 2B). However, none of the alkanes was detected immediately after irradiation in non-irradiated or irradiated beef samples (
Figure 2A). Two sulfur-containing compounds that were detected on day 0 in both non-irradiated and irradiated beef samples could also be found during all four days of storage (
Figure 2). Half of the ketones that were found in the beef samples during the experiment were detected on day 0, while the remaining ketones appeared over time (
Figure 2).
2.2. Analysis of Volatile Organic Compounds in Beef Immediately after Irradiation
Non-irradiated and irradiated beef samples were subjected to GC/MS analysis one hour after irradiation with accelerated electrons.
Figure 3 shows the chromatograms of an non-irradiated beef sample and a sample irradiated with 5 kGy.
The beef samples irradiated with 5 kGy demonstrate a greater number of peaks and an increase in the peak areas compared to the non-irradiated samples, which can be explained by the oxidation of lipids and proteins in the beef samples during irradiation. As can be seen from
Table 1 below, an increase in irradiation dose causes a greater number of VOCs to appear.
Most aldehydes (pentanal, hexanal, heptanal, octanal, nonanal), ketones (2,3-butandione, 2-pentanone), and alcohols (ethanol, 1-pentanol, 1-hexanol) show a dramatic increase in the concentration in the samples irradiated with 0.25 kGy and 0.5 kGy followed by a smoother rise in the concentration, while the irradiation with 5 kGy yields a concentration of VOCs that exceeds the control values by a factor of 1.9–7. In the case of 2-butanone ketone, an almost linear increase in concentration is observed with an increase in the irradiation dose. Importantly, while aldehydes, which are oxidation derivatives of lipids, were present in the non-irradiated beef samples (
Figure 3, highlighted in red), aldehydes butanal,2-methyl- and butanal,3-methyl- (
Figure 3, highlighted in purple), which are oxidation derivatives of proteins, were detected in the irradiated samples but not in non-irradiated ones.
It was found that the dose–effect relationship of VOC concentration was nonlinear, which, as our mathematical model shows, can result from two competing processes: the decomposition and accumulation of VOCs after irradiation as a result of the decomposition of other VOCs and biomacromolecules, such as fatty acids, proteins and carbohydrates. According to the model developed and published by our team [
33], a volatile compound
A present in the food sample at concentration
CA is caused by the ionizing radiation to decompose at a rate
kA, which is the number of compounds
A decomposed per unit of absorbed dose (Gy
−1). Under irradiation, compound
A can transform into other volatile organic compounds of different fractions, depending on the type of the resulting compound.
Suppose that during irradiation, only a fraction
q of the concentration of compound
A decomposes into volatile compound
B, with the value of
q in the dose range independent of the irradiation dose and determined only by the type of compound
B, which in turn also decomposes under the action of irradiation at rate
kB (Gy
−1). Thus, the
CB concentration of compound
B on the one hand increases as it is transformed from compound
A, and on the other hand decreases as a result of decomposition caused by irradiation. The change in the concentrations of compounds
A and
B with an increase in the irradiation dose can be expressed as follows:
Suppose that before irradiation, the concentration of compounds
and
. The solution of Equations (1) and (2) can be expressed as follows:
Since compound
A can not only decompose into different compounds but can also occur in the food sample during irradiation when another volatile compound or biomacromolecules decompose as a result of irradiation, the dose dependence of
CB concentration can be expressed as follows:
where the function
f(
D) depends linearly on the dose ranging from 0.25 kGy to 10 kGy. Suppose that
f(
D) depends linearly on the dose
D and
f(
D)
= gD, where
g (Gy
−1) is the proportionality factor.
Figure 4 shows the functions calculated using Formula (5), which approximate the dependencies of concentrations of different classes of VOCs, such as hexanal, 1-pentanol, 2-butanone and dimethyl sulfide, which are commonly identified in irradiated meat and fish.
Table 2 presents the approximation parameters obtained using Formula (5). High values of correlation coefficients and low standard error SE prove the validity of the mathematical model proposed by our team.
Figure 4 shows the typical behavior of VOC concentrations from the irradiation dose: a significant increase in the compound concentration in the beef samples irradiated with 5 kGy, which can be associated with a large number of chemical bond breaks in long organic chains leading to the formation of VOCs. All the dependencies presented in
Figure 4 have a local minimum, which is a sign of competition between decomposition and accumulation of VOCs. Hexanal and 1-pentanol have a local minimum in the region of 1 kGy, whereas a local minimum for dimethyl sulfide and 2-butanone shifts to around 250 Gy and 500 Gy, accordingly. Since the model was developed specifically for the pair of compounds in which one compound is an oxidation product of the other one, it does not take into account a wider range of channels through which each compound may, with a different probability, be formed and decomposed.
2.3. Analysis of Volatile Organic Compounds in Irradiated Beef Meat by GC/MS during Storage Time
During storage for four days, GC/MS analysis identified eight compounds more than on the day of irradiation. A total of 27 different volatile organic compounds were recorded: eight aldehydes (acetaldehyde (ethanal), propanal,2-methyl-, butanal,3-methyl-, pentanal, hexanal, heptanal, octanal, nonanal); six ketones (acetone, 2,3-butandione, 2-butanone, 2-pentanone, 2-butanone,3-hydroxy-, 2-butanone,3-hydroxy-); eight alcohols (ethanol, 1-butanol,3-methyl-, 1-butanol,2-methyl-, 1-propanol,2-methyl-, 1-pentanol, 1-hexanol, 1-octen-3-ol, 1-hexanol,2-ethyl-); two sulfur-containing volatiles (methanethiol, dimethyl sulfide); three alkanes (heptane, hexane, octane) and thiols. In similar works by other authors [
20] which analyzed VOCs in beef after irradiation, other classes of compounds such as pyrazines, alkenes, furans, and phenols were detected, which may be due to the different sensitivity of analytical instruments used for VOC identification and quantitative analysis, and beef properties, such as different intramuscular fat content [
34], or different types of fodder [
35].
Figure 5 shows the concentrations of VOCs in beef irradiated with different doses and non-irradiated beef samples that were identified during four days of storage. Various VOCs are plotted on the ordinate axis of the heat map, and the data on the abscissa axis are grouped by irradiation dose and storage time. The concentrations of VOCs (mg/kg) and the concentrations normalized to the maximum value of each compound are shown in
Figure 5A,B, respectively. Quantitative VOC monitoring data in non-irradiated and irradiated beef samples can be found in
Supplementary Table S1.
A number of compounds, such as 1-butanol,3-methyl-, 1-butanol,2-methyl-, propanol,2-methyl-, 1-octen-3-ol, 2-butanone,3-hydroxy-, 2-butanone,4-hydroxy-, heptane, hexane, and octane, were absent during the first 2 days of storage in both non-irradiated and irradiated beef samples. However, on day 2 after irradiation, all the abovementioned compounds were detected in the stored samples. It can also be noted that the maximum VOC concentrations were observed for most compounds in the samples irradiated with 5 kGy, which indicates a significant effect of the applied dose on the beef properties due to oxidation caused by ROS occurring in the samples during and after irradiation.
The heat maps show that different classes of compounds prevail depending on the day of storage. While a high content of aldehydes and methanethiol was observed from day 0 to day 2 after irradiation, and ketones as well as alcohols increased in the concentration from day 2 to day 3 and from day 2 to day 4, respectively, alkanes showed a dose-dependent growth from day 3 to day 4 after irradiation. Since VOCs determine beef odor, it can be concluded that the prevalence of aldehydes and sulfur-containing compounds causes beef to exude a specific odor immediately after irradiation. A specific smell of beef after 3–4 days of storage upon irradiation is the result of an increased concentration of alcohols and alkenes.
Figure 6A–I show the kinetics of the concentrations of different classes of volatile compounds, such as aldehydes, excluding acetaldehyde, as well as alcohols, ketones, alkanes, acetaldehyde, ethanol, methanethiol, dimethyl sulfide and butanal,3-methyl- in the beef samples irradiated with different doses as well as in non-irradiated samples. The time dependencies of the concentrations of acetaldehyde, butanal,3-methyl-, propanal,2-methyl, acetone, ethyl alcohol, and sulfur-containing compounds methanethiol and dimethyl sulfide in the beef samples are plotted individually, since these compounds show dramatically different behavior depending on the dose and storage time, which will be discussed further in this article.
Experimental results show that compounds within each individual class have the same dependencies of the concentrations on the time and dose, so for convenience, further discussion will take into account the mechanisms specific for each class.
2.3.1. Aldehydes
Despite the fact that the concentration of acetaldehyde in irradiated and non-irradiated samples decreased during storage (
Figure 6A), this compound was the predominant aldehyde in beef for all irradiation doses at different storage time as a result of decomposition of various fatty acids and alcohols present in beef [
36]. The nonlinear decrease in acetaldehyde concentration in irradiated and non-irradiated beef samples with time can be explained by its decomposition due to high reactivity and high oxidation rate in the presence of oxygen. The irradiation dose also had a nonlinear effect on the behavior of acetaldehyde throughout beef monitoring. It should also be noted that the acetaldehyde concentration plummeted on day 3 after irradiation after gradually subsiding within the first two days of storage.
Lipid Oxidation Aldehydes
According to previous studies [
20], degradation products of unsaturated fatty acids and straight-chain aldehydes, such as pentanal, hexanal, heptanal, octanal, and nonanal, are responsible for the specific odor of meat products after irradiation. Oxidation products of lipids showed a similar dependence of the concentration on the dose and storage time: an increase in concentration on day 1 and day 2 after irradiation followed by a sharp decline for the remaining period of monitoring (
Figure 6B). Interestingly, the time dependencies of both individual aldehydes and summary aldehyde concentration were clearly influenced by the irradiation dose: the higher the irradiation dose, the higher the maximum value of aldehyde concentrations recorded in the beef samples. Moreover, the peak chemical yield of aldehydes formed by lipid oxidation shifted towards longer observation time with an increase in the irradiation dose. It should be noted that the maximum and most prolonged effect of the release of aldehydes formed during lipid oxidation was observed in the beef samples irradiated with a dose of 5 kGy.
Protein Oxidation Aldehydes
An oxidation product of isoleucine amino acid branched aldehyde butanal,3-methyl [
35], a constituent of protein molecules, was not detected in the non-irradiated beef samples throughout the entire period of the experiment, which corresponds to similar experiments involving beef [
37]. In the samples irradiated with 0.25 kGy, butanal,3-methyl was only detected immediately after irradiation, and the concentration of this aldehyde was 0.08 mg/kg. In the samples irradiated with 0.5 kGy and 1 kGy, the concentration of butanal,3-methyl ranged from 0.06 mg/kg to 0.2 mg/kg, with the peak concentration observed on day 1 after irradiation, while no traces of butanal,3-methyl were detected further. The concentration of butanal,3-methyl in the samples irradiated with 5 kGy had a maximum on day 2 of monitoring, and after four days of storage, this aldehyde was still present in the sample, although its concentration decreased during the remaining two days of monitoring (
Figure 6C). Aldehyde butanal,2-methyl was only detected immediately after irradiation and it was not found during a further four days of monitoring.
The concentration of aldehyde propanal,2-methyl, formed both by oxidation of the alcohol propanol, amino acid isoleucine and amino acid valine [
38], decreased nonlinearly with the storage time. While propanal,2-methyl was initially present both in non-irradiated and all irradiated beef samples, its concentration subsided at a rate determined by the applied irradiation dose: the higher the dose, the more time was required for propanal,2-methyl to disappear. The concentration of propanal,2-methyl in the samples irradiated with 5 kGy had a maximum immediately after irradiation, and after four days of storage, this aldehyde was still present in the beef samples irradiated with 5 kGy, although its concentration decreased during the remaining four days of monitoring (
Figure 6C). It should be noted that while protein oxidation aldehydes registered in the beef samples irradiated with doses ranging from 0.25 kGy to 1 kGy were detected only within 2 days after irradiation, the beef samples irradiated with 5 kGy contained aldehydes during four days of observation (
Figure 6C).
The aldehydes, which are lipid oxidation derivatives detected in the irradiated beef samples, showed dramatically different dependence on the storage time compared to aldehydes, which are protein oxidation products: in contrast to a well-defined concentration peak of aldehydes formed from lipids during first two days after irradiation depending on the dose applied, after a slight growth in the concentration of the aldehydes formed from proteins during and immediately after irradiation, a gradual reduction in the value was observed throughout further four days of monitoring.
2.3.2. Alcohols
The total concentration of alcohols in all non-irradiated and irradiated beef samples increased with the storage time, and while it was marginally low immediately after irradiation and on day 1 of storage, the values rocketed on day 2 and continued growing during a further two days of monitoring (
Figure 6D). Straight-chain alcohols 1-pentanol and 1-hexanol are formed by oxidation of unsaturated fatty acids, similar to straight-chain aldehydes, while branched-chain alcohols 1-butanol,3-methyl-, 1-butanol,2-methyl-, 1-propanol,2-methyl-, 1-octen-3-ol and 1-hexanol,2-ethyl- can be accumulated as a result of the decomposition of proteins due to bacterial activity [
39] or other biochemical processes [
38].
The main contribution to the concentration of alcohols was made by ethanol (
Figure 6E), which is a product of glycogen hydrolysis initiated by microbial enzymatic transformations [
40]. The concentration of this compound in the non-irradiated beef samples increased exponentially from 0.033 mg/kg to 9.42 mg/kg during four days of storage, which is an indication of an increase in bacterial activity in the non-irradiated beef samples. A steady increase in ethanol concentration was observed in the beef samples irradiated with doses ranging from 0.25 kGy to 5 kGy, and the rate at which ethanol increased slowed as the irradiation dose went up, which is a sign of the inactivation of microorganisms by irradiation. Therefore, the higher the irradiation dose, the lower the concentration of microorganisms in beef and the lower the concentration of ethanol.
The concentration of straight-chain alcohols pentanol-1 and hexanol-1 showed a nonlinear dependence on storage time in the non-irradiated and irradiated beef samples. In the non-irradiated samples, the maximum concentrations of pentanol-1 and hexanol-1 (0.5–0.6 mg/kg) were observed on day 2 of storage. In all irradiated samples, pentanol-1 peaked on day 2–3 of storage for all irradiation doses. The concentration of hexanol-1 in irradiated beef samples increased nonlinearly with storage time (from 0.07–0.16 to 0.49–2.24 mg/kg) for all the doses. The higher the irradiation dose, the higher the concentration of this compound was detected in the beef samples, which is a sign of predominant accumulation of pentanol-1 and hexanol-1 due to oxidation caused by reactive oxygen species, whose concentration typically goes up with an increase in irradiation dose.
Despite its low concentration, 1-octen-3-ol (0.1–1.5 mg/kg), with a specific mushroom flavor odor, is a key flavor component of beef meat [
20]. Alcohol 1-octen-3-ol may be a degradation product of linoleic fatty acid or a reduction product of carbonyl compounds [
41]. In this study, 1-octen-3-ol was detected in all samples from two days of monitoring onwards: its concentration in the non-irradiated samples and the samples irradiated with 0.25 kGy and 0.5 kGy stood at the level of 0.1–0.4 mg/kg and did not change until the end of observation. In contrast, for doses of 1 kGy and 5 kGy an increase in the concentrations on day 2 of storage was three and six times higher compared to the control values, respectively, and further there was a decrease in the concentration of 1-octen-3-ol to 0.4 mg/kg and 0.7 mg/kg, for 1 kGy and 5 kGy, respectively, as this alcohol started to decompose.
In the remaining branched-chain alcohols 2-methyl-1-butanol, 3-methyl-1-butanol and 2-methyl-1-propanol, which are protein oxidation products, a nonlinear increase in the concentrations with the storage time was observed for all irradiation doses. It should be noted that branched-chain alcohols were absent in all beef samples during two days of monitoring (
Figure 6F). Therefore, a sharp growth in the concentration of these alcohols observed on day 2 to day 4 of storage after irradiation may be a sign of bacterial activity as well as oxidation occurring in the beef samples initiated by ROS present in both non-irradiated samples and to a greater extent in the irradiated samples throughout the observation period. At the same time, the concentration of alcohols formed by protein degradation detected in the beef samples irradiated with 5 kGy on day 2 of minoring was 10 times higher compared to ones in the beef samples irradiated with other doses (
Figure 6F). Thus, the concentration of alcohols from protein degradation is also an indicator of the effective microbial suppression in meat samples by irradiation.
2.3.3. Ketones
The total concentration of all ketones detected in the beef samples showed a dramatic increase on day 2 after irradiation for the doses ranging from 0 to 1 kGy reaching the peak of 25.5 mg/kg to 37.6 mg/kg depending on the dose, while the maximum concentration of ketones detected in the beef samples irradiated with 5 kGy was achieved on day 3 after irradiation and amounted to 35.6 mg/kg (
Figure 6G). The main contribution to the total concentration of ketones after irradiation was made by 2-butanone,3-hydroxy- and 2,3-butandione, which are responsible for a specific oily odor of meat [
20]. According to the literature [
42], 3-hydroxy-2-butanone and 2,3-butanedione are accumulated in meat products as a result of oxidation of the alcohol 2,3-butanediol, which is a product of fatty acid oxidation, through the decomposition of dicarbonyl and hydroxycarbonyl and through the autolysis of the polysaccharide glycogen, which is present in muscle tissue [
43]. Since no alcohol 2,3-butanediol was identified in any of the beef samples during the entire period of storage, 2-butanone,3-hydroxy- and 2,3-butandione were formed as a result of glycogen autolysis in the presence of hydrolytic enzymes catalyzing the decomposition of glycogen.
2.3.4. Sulfur-Containing Compounds
The sulfur-containing compounds methanethiol and dimethyl sulfide identified in the beef samples are derived from the degradation of sulfur-containing amino acids such as methionine, cysteine and cystine [
44], which are the building blocks of proteins. Dimethyl sulfide, detected in the non-irradiated and irradiated beef samples, was present at low concentrations (0.2 mg/kg or less) throughout the entire period of storage (
Figure 6H). Though the concentration of dimethyl sulfide is relatively low, it is responsible for a rather unpleasant odor that is frequently identified in irradiated meat products [
37]. It should be noted that the concentrations of this compound in beef samples irradiated with doses ranging from 0.25 kGy to 1 kGy were close to the control values throughout the observation time. The samples irradiated with 5 kGy showed a statistically significant 1.1–2.8-fold increase in dimethyl sulfide concentration compared to the controls throughout the entire period of monitoring.
The concentration of methanethiol, which has a putrid odor [
20], in the non-irradiated beef samples decreased exponentially from 3.0 mg/kg to 0.1 mg/kg during the whole observation time (
Figure 6H). In all irradiated beef samples, this compound showed a non-monotonic dependence of concentration on the storage time. Thus, in the samples irradiated with 0.25 kGy, the concentration of methanethiol decreased from 2.3 mg/kg to 0.5 mg/kg during the first two days to increase to 1.4 mg/kg on day 4 of monitoring. In the samples irradiated with 0.5 kGy and 1 kGy, the concentration of methanethiol was at the level of 2–3 mg/kg for the first two days and then demonstrated a nonlinear decrease with the storage time to 0.6 and 0.2 mg/kg, respectively.
In the samples irradiated with 5 kGy, for the first two days there was no significant change in the concentration of methanethiol (2.0–1.9 mg/kg), but on day 3 the concentration increased almost 1.5 times to decrease to 0.5–0.7 mg/kg on day 4 after irradiation. The monotonic decrease in methanethiol concentration throughout the storage time in the non-irradiated samples testifies to the gradual decomposition of methanethiol as a result of meat oxidation. Non-monotonic kinetics of methanethiol concentration in the irradiated samples is a sign of competition between accumulation of this compound triggered by protein oxidation, and decomposition of methanethiol as it is oxidized by reactive oxygen species.
2.3.5. Alkanes
The concentration of the identified alkanes heptane, hexane and octane, which are a combination of two organic radicals formed during the oxidation of fatty acids, increased nonlinearly in the irradiated beef samples with an increase in the storage time, and none of these compounds was detected in the non-irradiated beef samples (
Figure 6I). Alkanes were detected in the beef samples irradiated with doses ranging from 0.5 kGy to 5 kGy one day after irradiation, and the higher the dose, the higher concentration of these compounds. In the beef samples irradiated with 0.25 kGy, alkanes were detected two days after irradiation, and their concentration was at the level of the non-irradiated samples. Between day 2 and day 3 after irradiation, the concentration of alkanes in the samples irradiated with 0.25 kGy increased, and on day 3 after irradiation it was close to that of the samples irradiated with 0.5 kGy and 1 kGy. On day 4 after irradiation, the concentration of alkanes in the non-irradiated samples increased to the level of the samples irradiated with 0.25 kGy, so alkane concentration in the non-irradiated samples did not statistically differ from the concentration in the samples irradiated with 0.25 kGy. The highest concentration of alkanes (1.29 mg/kg) in the samples irradiated with 5 kGy registered on observation day 3 was dramatically different from the concentration of alkanes in all other irradiated samples, which ranged from 0.46 mg/kg to 0.53 mg/kg.
2.4. Oxidation Markers in Irradiated Beef during Storage
The impact of electron beams on biological tissue of meat as a result of both direct ionization of atoms and molecules by accelerated electrons as well as indirect action of electrons lead to biological dose–effect impacts in biomacromolecules. When exposed to accelerated electrons, chemical bonds in lipid and protein molecules break to initiate changes in molecule structure or charge distribution—polarity of molecules. The physical stage of radiation lasting for
s [
45] leads to the creation of ions and excited molecules, while ROS
1 species
, occurring as a result of water radiolysis, during physical and chemical stage of the irradiation
s actively interact with lipids, bringing about oxidative stress that initiates lipid peroxidation, triggering chain destructive reactions in the cell lipids and forming lipid radicals L*, LO*, as well as lipid hydroperoxides LOOH—ROS
2 [
24]. ROS
1 species also interact with amino acids, peptides and proteins, leading to both reversible and irreversible oxidation of proteins with the formation of protein peroxyl radicals (ROS
2), causing disruption, modification, carbonylation, oxidation and fragmentation of the primary, secondary and tertiary structure of protein molecules. The biochemical stage of irradiation lasts from
s to several days and even years [
45].
Figure 7 shows the stages of accelerated electron exposure for target lipids and proteins in meat samples.
Biochemical changes in the beef samples initiated by lipid and protein radicals keep occurring from a few seconds to a few days after irradiation. According to the experimental results obtained in our study, the dose–effect impact on the formation of lipid oxidation markers pentanal, hexanal, heptanal, octanal, and nonanal was detected throughout the four-day monitoring (
Figure 6B): the total concentration of aldehydes formed from lipid oxidation was higher with higher doses absorbed by the beef samples on each day of observation. Moreover, with an increase in the irradiation dose, the concentration peak of aldehydes occurred on later days of storage. It can be concluded that the direct action of accelerated electrons and indirect action of primary ROS
1 only affects the aldehyde concentration immediately after irradiation, while the secondary and tertiary ROS
2, whose concentration is higher the higher the irradiation dose, is evident throughout the entire period of storage. During storage in packaging that is not very tight, both non-irradiated and irradiated chilled meat is inevitably affected by background ROS
0 species, which are formed in the presence of oxygen. This explains why marginal concentrations of aldehydes formed from lipids can be traced in the non-irradiated beef samples.
Immediately after exposure to accelerated electrons, the protein oxidation aldehydes butanal,2-methyl, butanal,3-methyl and propanal,2-methyl were detected in the beef samples, and in contrast to the lipid oxidation markers that are present in the non-irradiated samples, butanal,2-methyl and butanal,3-methyl were detected only in the irradiated beef samples, with their concentration becoming commensurate with that of lipid-derived aldehydes only when irradiated with 5 kGy (
Figure 8). Aldehyde butanal,2-methyl was detected only immediately after irradiation and never traced in the further four days of monitoring. In contrast, the dose–effect impact on the formation of protein oxidation marker butanal,3-methyl was observed throughout the whole period of storage (
Figure 6C). Immediately after irradiation and during the first two days of monitoring, butanal,3-methyl concentration was higher with higher irradiation dose. While immediately after irradiation, butanal,3-methyl was detected in all the samples irradiated with different doses, after the first day of storage, it registered only in the samples irradiated with 0.5 kGy, 1 kGy and 5 kGy, and during the remaining days of monitoring, butanal,3-methyl was detected only in the beef samples irradiated with 5 kGy. Bearing in mind that butanal,3-methyl showed a clear dose dependence over time, this aldehyde can serve as a potential marker of protein oxidation in beef. Judging by the dose dependence of lipid and protein oxidation markers measured immediately after irradiation, it can be concluded that lipid oxidation is observed at lower doses than protein oxidation in beef. A similar effect of ROS on blood in vivo is observed when oxidation of blood cell lipids occurs at a lower concentration of ROS than carbonylation of protein molecules in blood cell membranes [
46].
During the monitoring, a clear dose dependence of the concentration of hydrocarbons formed by combining two lipid radicals was detected (
Figure 6I): on days 1–4 of the monitoring, and their concentration was higher the higher the dose absorbed by the beef samples. At the same time, immediately after irradiation, alkanes were not detected in either non-irradiated or in irradiated beef samples. Since the higher the dose, the more ROS
1 occur in the product and the more organic ROS
2 radicals are formed, it can be concluded that alkanes are markers of multistage oxidative processes of lipids in beef after irradiation.
Unlike irradiation markers of lipid and protein oxidation, ethanol was detected in all beef samples on all five days of monitoring. Beef monitoring after irradiation revealed a clear dependence of ethanol concentration on the irradiation dose: the higher the dose, the lower the rate at which ethanol concentration grew in the beef samples during storage and the lower the ethanol concentration recorded (
Figure 6E). Ethanol—a product of glycogen hydrolysis initiated by microbial enzymatic transformations—is a marker of bacterial content in meat during storage: the higher the ethanol concentration, the more bacteria are present in meat. Since ethanol was found in the beef samples irradiated with 5 kGy, though on day 4 its concentration was three times lower than non-irradiated samples, it is clear that even the highest irradiation dose did not fully inhibit bacteria in the beef samples. Still, the bacterial growth was suppressed to an extent deemed sufficient for extending the shelf life of the product. Since ethanol can be easily traced in meat products, it can be used as a marker of meat spoilage and testify whether the irradiation of meat was carried out correctly and homogeneously.
Summarizing all the results obtained during food irradiation research, it can be concluded that all VOCs are suitable markers of biochemical processes occurring in meat during storage: oxidative processes after irradiation, oxidation due to ROS0, which are always present in the product during storage, and microbial–enzymatic processes, to name a few. In this paragraph, we have highlighted only those VOCs that clearly showed a dose impact on the kinetics of VOC concentrations.
Figure 9 shows how irradiation markers appeared and disappeared throughout the four-day experiment.
Figure 9A shows the dose dependencies of the concentrations of aldehydes formed from lipids (red curve), the concentrations of aldehydes formed from proteins (purple curve), and butanal,3-methyl aldehyde concentration (blue curve) measured immediately after irradiation. The dependencies shown in
Figure 9A are calculated using Formula (5) and approximate the experimental data for aldehydes formed from lipids (
Figure 6B) and aldehydes formed from proteins (
Figure 6C).
Figure 9A zooms into day 0 measurements of aldehyde concentrations shown in
Figure 9B. As can be seen from
Figure 8, dose dependencies of the concentrations of aldehydes, which are lipid oxidation derivatives, as well as aldehydes resulting from the decomposition of leucine and isoleucine amino acids, were recorded immediately after irradiation, indicating the impact of direct and indirect action of accelerated electrons through the effect of ROS
1 on lipids and proteins (
Figure 9A). With an increase in the irradiation dose, the rate of increase in lipid aldehyde concentration significantly exceeds the rate of increase in protein aldehyde concentrations, which a sign of a high sensitivity of lipids to irradiation within the dose range of 0.25 kGy to 5 kGy compared to the sensitivity of proteins.
As the storage time passed, secondary and tertiary ROS led to the development of lipid oxidation, which was detected by the peak in the concentration of aldehydes formed from lipids from day 1 to day 2, after which the concentration of aldehydes gradually subsided to the values of non-irradiated samples. On day 3 and day 4 of monitoring, lipid oxidation products formed multistage oxidative products, hydrocarbons, whose peak concentration was observed on day 3 and 4 of monitoring. At the same time, the development of microbial–enzymatic processes on ethanol concentration was recorded, which indicated an increase in the number of microorganisms in beef. Overall, as storage time passed, oxidation products occurring as a result of irradiation replaced each other, with the maximum dose–effect impact being observed on day 1 and day 2 after irradiation, while bacterial content growth intensified on day 3 and day 4 of storage of beef samples at 4 °C (
Figure 9B). The aldehydes pentanal, hexanal, heptanal, octanal, and nonanal, and butanal,3-methyl, hydrocarbons, and ethanol found in irradiated beef during this study can serve as indicators of the impact of irradiation on beef: the content of VOCs can reveal the dose absorbed by the beef samples, as well as the ROS and microorganism concentration in beef after irradiation. Thus, the prolonged dose–effect impact of accelerated electrons on chemical parameters of meat during storage is crucial for selecting the optimal meat irradiation method.
2.5. Kinetics of the Concentration of Aldehydes in the Beef Samples Irradiated with Different Doses
We used concentrations of aldehydes pentanal, hexanal, heptanal, octanal, and nonanal, formed as a result of lipid oxidation to construct a mathematical model of the change in the aldehyde concentrations over time in the beef samples irradiated with different doses for developing a criterion for selection of optimal parameters of meat irradiation. Let us assume that the change in aldehyde concentration occurs as a result of both direct ionization of lipids by accelerated electrons and oxidation by ROS1 occurring as a result of water radiolysis in the beef samples. During the chemical stage of irradiation, interaction of lipids with ROS1 results in the formation of lipid peroxidation products hydroperoxides and lipid radicals, which continue to oxidize lipids throughout the observation time.
Let us assume that lipids are the main source of aldehydes, namely, lipid oxidation causes aldehyde to appear in the beef samples during storage, and aldehydes are initially absent in fresh tenderloin beef. Since background ROS0 can always be found in the stored beef samples as a result of the presence of oxygen, the aldehyde concentration in the non-irradiated samples was slightly above zero at the time of the first measurement. Let l(t) be the amount of non-oxidized lipids in the beef samples at time t; α, 1/s, be the rate constant of lipid oxidation due to both the direct action of radiation and the indirect action of ROS1 whose amount depends on the irradiation dose; let R0 be the number of ROS0, present in all beef samples during storage; let β be the coefficient of proportionality, namely, the number of ROS1 per unit of the dose absorbed by a beef sample.
Since the radical
is the most effective oxidant for lipids [
47], it can be assumed that only a part of the formed ROS
1 species is involved in lipid oxidation. Let
m =
m(
D) be the fraction of ROS
1 which oxidize lipids. Then the change in the number of non-oxidized lipids over time can be described by the following first order linear differential equation:
According to Equation (6), the amount of non-oxidized lipids decreases with time according to the exponential law:
where
lo is the initial amount of non-oxidized lipids in meat samples before irradiation with accelerated electrons.
Based on the fact that during irradiation of meat, not all lipids
lo were subjected to direct or indirect action of accelerated electrons, and also during storage not all lipids were oxidized under the action of organic radicals, the change in the number of oxidized lipids with time can be represented as:
where
k is the fraction of lipids that were affected by the direct ionization caused by accelerated electrons and also interacted with ROS
1 formed in meat samples. Hence,
k = k(
D) depends on the irradiation dose, because the higher the dose, the longer the irradiation time and the more electrons interact with the lipids, as well as more reactive oxygen species being formed in the meat samples both during irradiation.
Let us denote the quantity of aldehydes in the beef samples as
x. Since not all oxidized lipids form aldehydes, let us assume that only a fraction
q of the total amount of oxidized lipids
converted to aldehydes. Since the rate of increase in aldehydes is proportional to the rate of the increase in the quantity of oxidized lipids, the rate of the increase in the amount of aldehydes can be expressed as follows:
Taking into account (9), the equation describing the increase in aldehyde concentration due to lipid oxidation can be represented as follows:
According to the experimental data presented in
Figure 6B, the concentration of aldehydes after peaking on day 1 and day 2 of monitoring declined during the last two days of observation. Thus, Equation (10) is transformed into the following inhomogeneous differential equation of the first order:
where
γ, 1/s is the rate constant of aldehyde decomposition over time and
n =
n(
D) is the fraction of aldehydes that decompose into other compounds. If solved by the standard method and taking into account that initially there are no aldehydes, Equation (11) can be represented as:
Figure 10A shows the experimental data of summary concentration of the aldehydes pentanal, hexanal, heptanal, octanal, and nonanal in the beef samples irradiated with doses ranging from 0.25 kGy to 5 kGy during the storage time.
Figure 10A also presents an approximation of the experimental data calculated using Formula (12). In
Figure 10B, the calculated relationship (12) is presented for the concentration of aldehyde hexanal
CH, whose concentration mostly contributed to the total concentration of aldehydes produced by lipid oxidation.
According to the developed model, it was obtained that the expressions
are monotonic and dependent on the dose as follows:
where
are approximation coefficients with the values presented in
Table 3. In the case of the total aldehyde concentration approximation, the values of the model parameters are averages of all aldehydes from lipids. The values of approximation parameters calculated for the total aldehyde concentration using Formula (12) are averaged throughout all aldehyde group.
High values of correlation coefficients of R = 0.98–0.99 indicate the adequacy of the proposed mathematical model of the temporal relationship of the concentration of aldehydes formed from lipids in the beef samples irradiated with different doses. According to the model, the quantity of oxidized lipids that form aldehydes increases exponentially with an increase in the irradiation dose, which can be explained by the fact that the higher the dose, the more electrons directly acting on lipids as well as ROS1 occur as a result of water radiolysis, and the more lipids are oxidized during irradiation. According to the model, the amount of ROS, including background ROS0 and ROS1 appearing as a result of irradiation, expressed as m(D), monotonically decreases with a higher irradiation dose, since a higher dose can increase the probability of the interaction of radicals with other organic molecules. Moreover, the chances of radical neutralization may be higher with the increase in the irradiation dose. As the model shows, the quantity of aldehydes that decompose into other compounds expressed as decreases with a higher irradiation dose, which can be explained by the fact that the probability of aldehydes entering various chemical reactions that cause them to decompose is influenced by other biochemical processes occurring in irradiated beef during storage.