The Application of Natural Carotenoids in Multiple Fields and Their Encapsulation Technology: A Review
Abstract
:1. Introduction
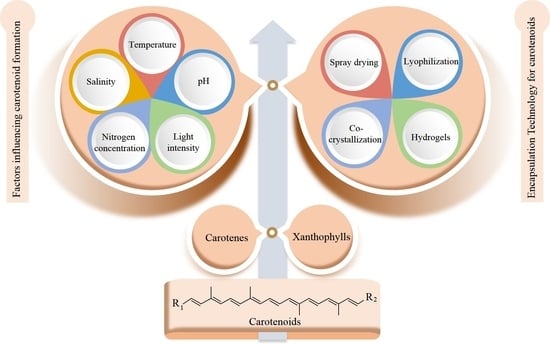
2. Sources, Structure, and Influencing Factors of Carotenoids
2.1. Sources and Structure of Carotenoids
2.2. Factors Influencing Carotenoid Formation
2.2.1. Temperature
2.2.2. pH and Light Intensity
2.2.3. Nitrogen Concentration
2.2.4. Salinity
3. Applications of Carotenoids in Multiple Fields
3.1. Application of Carotenoids in the Pharmaceutical Field
3.1.1. Application of Carotenoids in the Prevention of Respiratory Diseases
3.1.2. Application of Carotenoids in the Prevention of Ocular Complications
3.1.3. Application of Carotenoids in the Prevention of Chronic Ailments
3.1.4. Application of Carotenoids in the Prevention of Neurodegenerative Diseases
3.2. Application of Carotenoids in the Food and Feed Field
3.3. Application of Carotenoids in the Cosmetics Field
4. Encapsulation Technology
4.1. Spray-Drying Encapsulation
4.1.1. Emulsions by Spray-Drying
4.1.2. Liposomes by Spray-Drying
4.2. Lyophilization Encapsulation
4.3. Co-Crystallization Encapsulation
4.4. Hydrogel Encapsulation
5. Future Prospects
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocha, M.A.M.; Sandoval, C.A.M. Designing for sustainable development in a remote Mexican community. Interactions 2014, 21, 76–79. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Khoo, H.E.; Kong, K.W.; Prasad, K.N.; Galanakis, C.M. 8—Extraction of carotenoids and applications. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 259–288. [Google Scholar]
- Ursache, F.M.; Andronoiu, D.G.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleţ, M.; Dumitraşcu, L.; Botez, E.; Râpeanu, G.; Stănciuc, N. Valorizations of carotenoids from sea buckthorn extract by microencapsulation and formulation of value-added food products. J. Food Eng. 2018, 219, 16–24. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Joshi, K.; Kumar, P.; Kataria, R. Microbial carotenoid production and their potential applications as antioxidants: A current update. Process Biochem. 2023, 128, 190–205. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.-H.; Rajesh Banu, J.; Kumar, G. Technological advances in the production of carotenoids and their applications—A critical review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Chun, B.S. Optimization of carotenoids and antioxidant activity of oils obtained from a co-extraction of citrus (Yuzu ichandrin) by-products using supercritical carbon dioxide. Biomass Bioenergy 2017, 106, 1–7. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Roll Zimmer, T.B.; Barboza Mendonça, C.R.; Zambiazi, R.C. Methods of protection and application of carotenoids in foods—A bibliographic review. Food Biosci. 2022, 48, 101829. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Strati, I.F.; Sinanoglou, V.J.; Kora, L.; Miniadis-Meimaroglou, S.; Oreopoulou, V. Carotenoids from Foods of Plant, Animal and Marine Origin: An Efficient HPLC-DAD Separation Method. Foods 2012, 1, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.u.d.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Muni, M.; Mitra, S.; Emran, T.B.; Chandran, D.; Das, R.; Rauf, A.; Safi, S.Z.; Chidambaram, K.; Dhawan, M.; et al. Recent advances in respiratory diseases: Dietary carotenoids as choice of therapeutics. Biomed. Pharmacother. 2022, 155, 113786. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, Y.; Zhou, D.; Wang, J.; Li, J.; Sun, J.; Feng, Y.; Xin, F.; Zhang, W. Advances in the synthesis of three typical tetraterpenoids including β-carotene, lycopene and astaxanthin. Biotechnol. Adv. 2022, 61, 108033. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive compounds in microalgae and their potential health benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Singh, D.P.; Khattar, J.S.; Rajput, A.; Chaudhary, R.; Singh, R. High production of carotenoids by the green microalga Asterarcys quadricellulare PUMCC 5.1.1 under optimized culture conditions. PLoS ONE 2019, 14, e0221930. [Google Scholar] [CrossRef]
- Dyaa, A.; Soliman, H.; Abdelrazak, A.; Samra, B.N.; Khojah, E.; Ahmed, A.F.; El-Esawi, M.A.; Elsayed, A. Optimization of Carotenoids Production from Rhodotorula sp. Strain ATL72 for Enhancing Its Biotechnological Applications. J. Fungi 2022, 8, 160. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Z.; Chen, F. Kinetic modeling of lutein production by heterotrophic Chlorella at various pH and temperatures. Mol. Nutr. Food Res. 2006, 50, 763–768. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Chagas, A.L.; Rios, A.O.; Jarenkow, A.; Marcílio, N.R.; Ayub, M.A.Z.; Rech, R. Production of carotenoids and lipids by Dunaliella tertiolecta using CO2 from beer fermentation. Process Biochem. 2015, 50, 981–988. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Guimarães, A.A.C.; Rocha, L.V.F.; Winterburn, J.; Santos-Ebinuma, V.d.C.; Pereira, J.F.B. Improvement of carotenoids production from Rhodotorula glutinis CCT-2186. Biochem. Eng. J. 2021, 165, 107827. [Google Scholar] [CrossRef]
- Bohne, F.; Linden, H. Regulation of carotenoid biosynthesis genes in response to light in Chlamydomonas reinhardtii. Biochim. Et Biophys. Acta (BBA)—Gene Struct. Expr. 2002, 1579, 26–34. [Google Scholar] [CrossRef]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Barthelemy, V.; Schreiber, N.; Brun, P.; Lebouvier, N.; Jauffrais, T. Effects of Nitrogen Availability on the Antioxidant Activity and Carotenoid Content of the Microalgae Nephroselmis sp. Mar. Drugs 2020, 18, 453. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Meng, C.; Chen, Y.C.; Ahmed, F.; Mangott, A.; Schenk, P.M.; Li, Y. Comparison of astaxanthin accumulation and biosynthesis gene expression of three Haematococcus pluvialis strains upon salinity stress. J. Appl. Phycol. 2015, 27, 1853–1860. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, J.; He, W.; Zhang, H.Y. Influence of initial check, information exchange, final accuracy check, reaction information nursing on the psychology of elderly with lung cancer. World J. Clin. Cases 2024, 12, 737–745. [Google Scholar] [CrossRef]
- Schulze, F.; Gao, X.; Virzonis, D.; Damiati, S.; Schneider, M.R.; Kodzius, R. Air Quality Effects on Human Health and Approaches for Its Assessment through Microfluidic Chips. Genes 2017, 8, 244. [Google Scholar] [CrossRef]
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Aichour, M.T.E.; Alam, K.; et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Enilari, O.; Sinha, S. The Global Impact of Asthma in Adult Populations. Ann. Glob. Health 2019, 85, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, L.; Lawrence, J.C.; Tan, L. Utilization of Biopolymer-Based Lutein Emulsion as an Effective Delivery System to Improve Lutein Bioavailability in Neonatal Rats. Nutrients 2024, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, W.; Du, J. Association between dietary carotenoid intakes and the risk of asthma in adults: A cross-sectional study of NHANES, 2007–2012. BMJ Open 2022, 12, e052320. [Google Scholar] [CrossRef] [PubMed]
- Grievink, L.; de Waart, F.G.; Schouten, E.G.; Kok, F.J. Serum carotenoids, alpha-tocopherol, and lung function among Dutch elderly. Am. J. Respir. Crit. Care Med. 2000, 161, 790–795. [Google Scholar] [CrossRef]
- Ng, T.P.; Niti, M.; Yap, K.B.; Tan, W.C. Dietary and supplemental antioxidant and anti-inflammatory nutrient intakes and pulmonary function. Public Health Nutr. 2014, 17, 2081–2086. [Google Scholar] [CrossRef]
- Schünemann, H.J.; McCann, S.; Grant, B.J.; Trevisan, M.; Muti, P.; Freudenheim, J.L. Lung function in relation to intake of carotenoids and other antioxidant vitamins in a population-based study. Am. J. Epidemiol. 2002, 155, 463–471. [Google Scholar] [CrossRef]
- Dai, M.; Li, C.; Yang, Z.; Sui, Z.; Li, J.; Dong, P.; Liang, X. The Astaxanthin Aggregation Pattern Greatly Influences Its Antioxidant Activity: A Comparative Study in Caco-2 Cells. Antioxidants 2020, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.K.; Law, B.Y.; Lo, A.C. Lutein Attenuates Both Apoptosis and Autophagy upon Cobalt (II) Chloride-Induced Hypoxia in Rat Műller Cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Madrid, R.; Carballo-Uicab, V.M.; Cárdenas-Conejo, Y.; Aguilar-Espinosa, M.; Siva, R. 1—Overview of carotenoids and beneficial effects on human health. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–40. [Google Scholar]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 7, Cd000254. [Google Scholar] [CrossRef]
- Toragall, V.; Srirangam, P.; Jayapala, N.; Baskaran, V. Lutein encapsulated oleic—Linoleic acid nanoemulsion boosts oral bioavailability of the eye protective carotenoid lutein in rat model. Mater. Today Commun. 2021, 28, 102522. [Google Scholar] [CrossRef]
- Sluijs, I.; Cadier, E.; Beulens, J.W.J.; van der Schouw, Y.T.; Spijkerman, A.M.W.; van der Schouw, Y.T. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [CrossRef]
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and cardiovascular health 2. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Tan, H.-L.; Thomas-Ahner, J.M.; Pearl, D.K.; Erdman, J.W., Jr.; Moran, N.E.; Clinton, S.K. Dietary Tomato and Lycopene Impact Androgen Signaling- and Carcinogenesis-Related Gene Expression during Early TRAMP Prostate Carcinogenesis. Cancer Prev. Res. 2014, 7, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Cooperstone, J.L.; Schwartz, S.J. 20—Recent Insights Into Health Benefits of Carotenoids. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 473–497. [Google Scholar]
- Yang, J.; Fan, H.; Jiang, B.; Li, R.; Fan, J.; Li, B.; Ge, J.; Pan, S.; Liu, F. Excipient emulsion prepared with pectin and sodium caseinate to improve the bioaccessibility of carotenoids in mandarin juice: The effect of emulsifier and polymer concentration. Food Chem. X 2023, 20, 100909. [Google Scholar] [CrossRef]
- Thakur, D.; Jain, A.; Ghoshal, G.; Shivhare, U.S.; Katare, O.P. Microencapsulation of β-Carotene Based on Casein/Guar Gum Blend Using Zeta Potential-Yield Stress Phenomenon: An Approach to Enhance Photo-stability and Retention of Functionality. AAPS PharmSciTech 2017, 18, 1447–1459. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; Reig García-Galbis, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the Valuable Carotenoids for the Large-Scale Production by Marine Microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Wu, Y.; Zhang, Y.; Wang, Z.; Liu, X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol. Nutr. Food Res. 2015, 59, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Chopra, K. Lycopene abrogates Aβ(1-42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J. Nutr. Biochem. 2015, 26, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, F.; Hu, X.; Chen, J.; Wen, X.; Sun, Y.; Liu, Y.; Tang, R.; Zheng, K.; Song, Y. Inhibition of inflammation by astaxanthin alleviates cognition deficits in diabetic mice. Physiol. Behav. 2015, 151, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zheng, Y.; Guo, M.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martínez, M.-A. Oxidative stress, the blood-brain barrier and neurodegenerative diseases: The critical beneficial role of dietary antioxidants. Acta Pharm. Sin. B 2023, 13, 3988–4024. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.; Garkal, A.; Kudarha, R.; Hebbar, S.; Mutalik, S.; Mehta, T. Carotenoid containing cationic nanoparticles for effective therapy for suppressing oxidative stress: An intranasal approach. OpenNano 2023, 13, 100172. [Google Scholar] [CrossRef]
- Galaup, P.; Sutthiwong, N.; Leclercq-Perlat, M.-N.; Valla, A.; Caro, Y.; Fouillaud, M.; Guérard, F.; Dufossé, L. First isolation of Brevibacterium sp. pigments in the rind of an industrial red-smear-ripened soft cheese. Int. J. Dairy Technol. 2015, 68, 144–147. [Google Scholar] [CrossRef]
- Tabbers, M.M.; DiLorenzo, C.; Berger, M.Y.; Faure, C.; Langendam, M.W.; Nurko, S.; Staiano, A.; Vandenplas, Y.; Benninga, M.A. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Maróstica Júnior, M.R.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Medeiros, A.K.d.O.C.; Gomes, C.d.C.; Amaral, M.L.Q.d.A.; Medeiros, L.D.G.d.; Medeiros, I.; Porto, D.L.; Aragão, C.F.S.; Maciel, B.L.L.; Morais, A.H.d.A.; Passos, T.S. Nanoencapsulation improved water solubility and color stability of carotenoids extracted from Cantaloupe melon (Cucumis melo L.). Food Chem. 2019, 270, 562–572. [Google Scholar] [CrossRef]
- Himanath, G.; Shruthy, R.; Preetha, R.; Sreejit, V. Nanoemulsion with Coconut Oil and Soy Lecithin as a Stable Delivery System for Lycopene and Its Incorporation into Yogurt to Enhance Antioxidant Properties and Maintain Quality. ACS Food Sci. Technol. 2021, 1, 1538–1549. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Silva, T.R.E.; Silva, L.C.F.J.; de Queiroz, A.C.; Alexandre Moreira, M.S.; de Carvalho Fraga, C.A.; de Menezes, G.C.A.; Rosa, L.H.; Bicas, J.; de Oliveira, V.M.; Duarte, A.W.F. Pigments from Antarctic bacteria and their biotechnological applications. Crit. Rev. Biotechnol. 2021, 41, 809–826. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, Z.; Mehrgan, M.S.; Khayatzadeh, J.; Shekarabi, S.P.H.; Tabrizi, M.H. Dietary green-synthesized curcumin-mediated zinc oxide nanoparticles promote growth performance, haemato-biochemical profile, antioxidant status, immunity, and carcass quality in Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2023, 32, 101717. [Google Scholar] [CrossRef]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Thevamirtha, C.; Balasubramaniyam, A.; Srithayalan, S.; Selvakumar, P.M. An Insight into the antioxidant activity of the facial cream, solid soap and liquid soap made using the carotenoid extract of palmyrah (Borassus flabellifer) fruit pulp. Ind. Crops Prod. 2023, 195, 116413. [Google Scholar] [CrossRef]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Zhuang, D.; He, N.; Khoo, K.S.; Ng, E.-P.; Chew, K.W.; Ling, T.C. Application progress of bioactive compounds in microalgae on pharmaceutical and cosmetics. Chemosphere 2022, 291, 132932. [Google Scholar] [CrossRef]
- Lee, H.S.; Sung, D.K.; Kim, S.H.; Choi, W.I.; Hwang, E.T.; Choi, D.J.; Chang, J.H. Controlled release of astaxanthin from nanoporous silicified-phospholipids assembled boron nitride complex for cosmetic applications. Appl. Surf. Sci. 2017, 424, 15–19. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Xianquan, S.; Shi, J.; Kakuda, Y.; Yueming, J. Stability of Lycopene During Food Processing and Storage. J. Med. Food 2005, 8, 413–422. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Schuchmann, H.P.; Engel, R.; Walz, E.; Briviba, K. Encapsulation of Carotenoids. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V., Eds.; Springer: New York, NY, USA, 2010; pp. 211–252. [Google Scholar]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Eun, J.B.; Maruf, A.; Das, P.R.; Nam, S.H. A review of encapsulation of carotenoids using spray drying and freeze drying. Crit. Rev. Food Sci. Nutr. 2020, 60, 3547–3572. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef]
- Santos, P.D.d.F.; Rubio, F.T.V.; Balieiro, J.C.d.C.; Thomazini, M.; Favaro-Trindade, C.S. Application of spray drying for production of microparticles containing the carotenoid-rich tucumã oil (Astrocaryum vulgare Mart.). LWT 2021, 143, 111106. [Google Scholar] [CrossRef]
- Moraes, M.; Carvalho, J.M.P.; Silva, C.R.; Cho, S.; Sola, M.R.; Pinho, S.C. Liposomes encapsulating beta-carotene produced by the proliposomes method: Characterisation and shelf life of powders and phospholipid vesicles. Int. J. Food Sci. Technol. 2013, 48, 274–282. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Moraes, I.C.F.; Favaro-Trindade, C.S. Reducing carotenoid loss during storage by co-encapsulation of pequi and buriti oils in oil-in-water emulsions followed by freeze-drying: Use of heated and unheated whey protein isolates as emulsifiers. Food Res. Int. 2020, 130, 108901. [Google Scholar] [CrossRef]
- Kaur, P.; Elsayed, A.; Subramanian, J.; Singh, A. Encapsulation of carotenoids with sucrose by co-crystallization: Physicochemical properties, characterization and thermal stability of pigments. LWT 2021, 140, 110810. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-carotene in alginate-based hydrogel beads: Impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef]
- Bratovcic, A.; Suljagic, J. Micro- and nano-encapsulation in food industry. Croat. J. Food Sci. Technol. 2019, 11, 113–121. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Ribeiro, M.L.F.F.; Roos, Y.H.; Ribeiro, A.P.B.; Nicoletti, V.R. Effects of maltodextrin content in double-layer emulsion for production and storage of spray-dried carotenoid-rich microcapsules. Food Bioprod. Process. 2020, 124, 208–221. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Chu, L.; Xia, Q. Liposome-chitosan hydrogel bead delivery system for the encapsulation of linseed oil and quercetin: Preparation and in vitro characterization studies. LWT 2020, 117, 108615. [Google Scholar] [CrossRef]
- Marín, D.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Encapsulation of food waste compounds in soy phosphatidylcholine liposomes: Effect of freeze-drying, storage stability and functional aptitude. J. Food Eng. 2018, 223, 132–143. [Google Scholar] [CrossRef]
- Gu, Z.; Zhao, H.; Song, Y.; Kou, Y.; Yang, W.; Li, Y.; Li, X.; Ding, L.; Sun, Z.; Lin, J.; et al. PEGylated-liposomal astaxanthin ameliorates Aβ neurotoxicity and Alzheimer-related phenotypes by scavenging formaldehyde. J. Control Release 2024, 366, 783–797. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; Paris, C.; Guedon, E.; Linder, M.; Desobry, S. Liposomal nanodelivery systems using soy and marine lecithin to encapsulate food biopreservative nisin. LWT Food Sci. Technol. 2015, 62, 341–349. [Google Scholar] [CrossRef]
- Thompson, A.K.; Mozafari, M.R.; Singh, H.J.L. The properties of liposomes produced from milk fat globule membrane material using different techniques. Lait 2007, 87, 349–360. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Chaves, M.A.; Dacanal, G.C.; Pinho, S.C. High-shear wet agglomeration process for enriching cornstarch with curcumin and vitamin D3 co-loaded lyophilized liposomes. Food Res. Int. 2023, 169, 112809. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, Z.; Wang, S.; Chen, J.; Liu, Q.; Tianle, H.; Hai, L.; Lu, R.; Wu, Y. Berberine and folic acid co-modified pH-sensitive cascade-targeted PTX-liposomes coated with Tween 80 for treating glioma. Bioorganic Med. Chem. 2022, 69, 116893. [Google Scholar] [CrossRef] [PubMed]
- Prathyusha, E.; Prabakaran, A.; Ahmed, H.; Dethe, M.R.; Agrawal, M.; Gangipangi, V.; Sudhagar, S.; Krishna, K.V.; Dubey, S.K.; Pemmaraju, D.B.; et al. Investigation of ROS generating capacity of curcumin-loaded liposomes and its in vitro cytotoxicity on MCF-7 cell lines using photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 40, 103091. [Google Scholar] [CrossRef]
- Fang, S.; Niu, Y.; Zhang, W.; Zhang, Y.; Yu, L.; Zhang, Y.; Li, X. Liposome-like nanocapsules of dual drug-tailed betaine for cancer therapy. Int. J. Pharm. 2015, 493, 460–465. [Google Scholar] [CrossRef]
- Mattos, M.V.C.d.V.d.; Michelon, M.; Burkert, J.F.d.M. Production and stability of food-grade liposomes containing microbial carotenoids from Rhodotorula mucilaginosa. Food Struct. 2022, 33, 100282. [Google Scholar] [CrossRef]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the flavonoid quercetin with chitosan-coated nano-liposomes. LWT Food Sci. Technol. 2017, 85, 37–44. [Google Scholar] [CrossRef]
- Luo, W.-C.; Zhang, W.; Kim, R.; Chong, H.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of controlled ice nucleation and lyoprotectants on nanoparticle stability during Freeze-drying and upon storage. Int. J. Pharm. 2023, 641, 123084. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Lenart, A. Freeze-Drying—Application in Food Processing and Biotechnology—A Review. Pol. J. Food Nutr. Sci. 2011, 61, 165–171. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A method for pomegranate seed application in food industries: Seed oil encapsulation. Food Bioprod. Process. 2012, 90, 639–652. [Google Scholar] [CrossRef]
- Sharma, A.; Dhiman, A.; Attri, S. Encapsulation of extracted carotenoids of Cucurbita maxima through lyophilization. Pigment Resin Technol. 2020. ahead-of-print. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Navarro, A. Physicochemical properties and stability of sucrose/glucose agglomerates obtained by cocrystallization. J. Food Process Eng. 2018, 41, e12901. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Hartel, R.W. Co-crystallization of Sucrose at High Concentration in the Presence of Glucose and Fructose. J. Food Sci. 2002, 67, 1797–1802. [Google Scholar] [CrossRef]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Tavsanli, B.; Okay, O. Mechanically robust and stretchable silk/hyaluronic acid hydrogels. Carbohydr. Polym. 2019, 208, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Chen, L.; Tong, Q.; McClements, D.J. Designing hydrogel particles for controlled or targeted release of lipophilic bioactive agents in the gastrointestinal tract. Eur. Polym. J. 2015, 72, 698–716. [Google Scholar] [CrossRef]
- Hamdi, M.; Feki, A.; Bardaa, S.; Li, S.; Nagarajan, S.; Mellouli, M.; Boudawara, T.; Sahnoun, Z.; Nasri, M.; Nasri, R. A novel blue crab chitosan/protein composite hydrogel enriched with carotenoids endowed with distinguished wound healing capability: In vitro characterization and in vivo assessment. Mater. Sci. Eng. C 2020, 113, 110978. [Google Scholar] [CrossRef]
- Dias, C.; Nobre, B.P.; Santos, J.A.L.; Lopes da Silva, T.; Reis, A. Direct lipid and carotenoid extraction from Rhodosporidium toruloides broth culture after high pressure homogenization cell disruption: Strategies, methodologies, and yields. Biochem. Eng. J. 2022, 189, 108712. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits-A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef]
- Scaglia, B.; D’Incecco, P.; Squillace, P.; Dell’Orto, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Moon, S.H.; Keum, Y.-S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valuation of Citrus reticulata (kinnow) peel for the extraction of lutein using ultrasonication technique. Biomass Convers. Biorefinery 2021, 11, 2157–2165. [Google Scholar] [CrossRef]
- Chandra Roy, V.; Ho, T.C.; Lee, H.-J.; Park, J.-S.; Nam, S.Y.; Lee, H.; Getachew, A.T.; Chun, B.-S. Extraction of astaxanthin using ultrasound-assisted natural deep eutectic solvents from shrimp wastes and its application in bioactive films. J. Clean. Prod. 2021, 284, 125417. [Google Scholar] [CrossRef]
- Murador, D.C.; De Souza Mesquita, L.M.; Neves, B.V.; Braga, A.R.C.; Martins, P.L.G.; Zepka, L.Q.; De Rosso, V.V. Bioaccessibility and cellular uptake by Caco-2 cells of carotenoids and chlorophylls from orange peels: A comparison between conventional and ionic liquid mediated extractions. Food Chem. 2021, 339, 127818. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Lim, J.W.; Rawindran, H.; Chai, Y.H.; Tan, I.S.; Fui Chin, B.L.; Kiew, P.L. The potential of waste chicken feather protein hydrolysate as microalgae biostimulant using organic fertilizer as nutrients source. IOP Conf. Ser. Earth Environ. Sci. 2022, 1074, 012028. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Chin, B.L.F.; Shuit, S.H.; Lim, S.; Pang, Y.L.; Kiew, P.L. Abiotic stress as a dynamic strategy for enhancing high value phytochemicals in microalgae: Critical insights, challenges and future prospects. Biotechnol. Adv. 2024, 70, 108280. [Google Scholar] [CrossRef] [PubMed]






| Encapsulation Technique | Major Carotenoids | Wall Material | Encapsulation Efficiency (%) | Particle Size (μm) | Stability | Bioaccessibility | Reference |
|---|---|---|---|---|---|---|---|
| Spray-drying | Tucumã oil (rich in carotenoids) | Gum Arabic | 120 °C inlet air temperature and different oil proportion (g/kg) 100 (g/kg): 90.6 ± 0.3% 200 (g/kg): 74.2 ± 0.3% 300 (g/kg): 44.4 ± 0.3% | 0.6~82.2 μm | Spray-dried encapsulation resulted in at least a 142-fold increase in the thermal stability of tucumã oil and a significant increase in oxidative stability and retention after encapsulation. | After complete digestion, the total release of total carotenoids in tucumã oil microparticles reached 64%. | [81] |
| β-carotene | Hydrogenated phosphatidylcholine and sucrose | - | 1500 nm | More than 90% of the β-carotene was preserved when refrigerated in a vacuum for 60 days. The liposome dispersion maintained its average size, polydispersity index, and zeta potential over 100 days. After 60 days, the degradation of the encapsulated carotene was minimal, and the color of the dispersion was preserved. | - | [82] | |
| Lyophilization | Buriti and pequi oils (rich in carotenoids) | Whey protein isolate (WPI) | - | 0.88 ± 0.03~2.33 ± 0.02 μm | Formulations prepared with WPI (heated and unheated) and then freeze-dried enhanced carotenoid protection (48–30% retention after 50 days of storage) and improved the oxidation stability of the oil (OSI 51 and 46 h) compared to unencapsulated materials (30% total carotenoid retention after 32 days of storage, OSI 20.5 h). | - | [83] |
| Co-crystallization | β-carotene | Sucrose | 77.58% | - | Co-crystallization significantly improved the overall stability of carotenoids. | - | [84] |
| Hydrogels | β-carotene | Alginate | - | 0.5% alginate encapsulated: 285 μm 1% alginate encapsulated: 660 μm | The hydrogel beads partially protected β-carotene from chemical degradation, the extent of which depended on alginate levels within the beads. | The bioavailability of β-carotene encapsulated in hydrogels was relatively low. This might be due to some β-carotene molecules remaining in undigested fat droplets within the hydrogel. Second, fewer free fatty acids and monacylglycerol were available to form mixed micelles, reducing intestinal absorption. | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, Y.; Zhang, H.; Ding, Z.; Han, J. The Application of Natural Carotenoids in Multiple Fields and Their Encapsulation Technology: A Review. Molecules 2024, 29, 967. https://doi.org/10.3390/molecules29050967
Li Y, Zhao Y, Zhang H, Ding Z, Han J. The Application of Natural Carotenoids in Multiple Fields and Their Encapsulation Technology: A Review. Molecules. 2024; 29(5):967. https://doi.org/10.3390/molecules29050967
Chicago/Turabian StyleLi, Yinglan, Yanna Zhao, Huaizhen Zhang, Zhuang Ding, and Jun Han. 2024. "The Application of Natural Carotenoids in Multiple Fields and Their Encapsulation Technology: A Review" Molecules 29, no. 5: 967. https://doi.org/10.3390/molecules29050967
APA StyleLi, Y., Zhao, Y., Zhang, H., Ding, Z., & Han, J. (2024). The Application of Natural Carotenoids in Multiple Fields and Their Encapsulation Technology: A Review. Molecules, 29(5), 967. https://doi.org/10.3390/molecules29050967