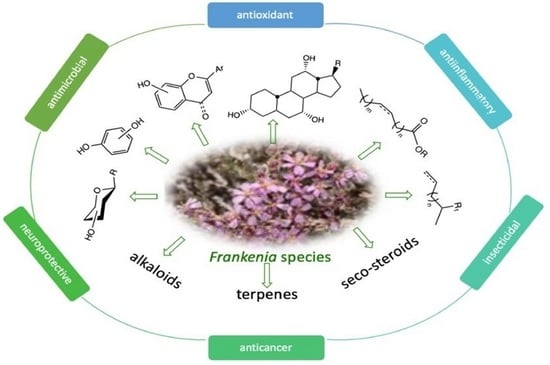
Phytochemical Profiles and Biological Activities of Frankenia Species: A Review
Abstract
:1. Introduction
2. Phytochemical Profile of Frankenia Plants
2.1. Polyphenols
2.1.1. Phenolics
2.1.2. Flavonoids
2.1.3. Lignans
2.1.4. Coumarins
2.2. Alkaloids
2.3. Terpenoids
2.3.1. Monoterpenes
2.3.2. Diterpenes
2.3.3. Sesquiterpenes
2.4. Steroids
2.5. Alkanes and Alkenes
2.6. Fatty Acids and Esters
2.7. Other Compounds
2.8. Phytochemical Outcome
3. Biological Activities of Frankenia Plants
3.1. In Traditional Medicine
3.2. In Vitro Biological Activities
3.2.1. Antioxidant Activity
| Frankenia Species | Extract/ Fraction | Organ | Assay | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | CCA | ICA | ORAC | β-Carotene | MCA | ||||
| F. laevis | aqueous acetone | AP | IC50 = 0.12 mg/mL | IC50 = 0.18 mg/mL | n.d | IC50 = 0.44 mg/mL | IC50 > 1 mg/mL | n.d | n.d | n.d | [17] |
| methanol | AP | EC50 = 0.25 mg/mL | EC50 = 0.65 mg/mL | EC50 = 0.51 mg/mL | EC50 = 0.78 mg/mL | EC50 > 1 mg/mL | n.d | n.d | n.d | [13] | |
| dichloromethane | EC50 > 1 mg/mL | EC50 > 1 mg/mL | EC50> 1 mg/mL | EC50 > 1 mg/mL | EC50 = 0.76 mg/mL | n.d | n.d | n.d | |||
| ethanol | Sh | IC50 = 48.3 µg/mL | IC50 = 93.4 µg/mL | n.d | n.d | IC50 = 240 µg/mL | n.d | n.d | n.d | [41] | |
| F. pulverulenta | aqueous acetone | AP | IC50 = 0.10 mg/mL | IC50 = 0.15 mg/mL | n.d | IC50 = 0.30 mg/mL | IC50 = 0.50 mg/mL | n.d | n.d | n.d | [17] |
| ethyl acetate | Sh | 586 mg TE/g E | 1453 mg TE/g E | n.d | n.d | n.d | 821 mg TE/g E | n.d | 37 mg EDTA/g E | [19] | |
| R | 750 mg TE/g E | 1319 mg TE/g E | n.d | n.d | n.d | 1054 mg TE/g E | n.d | 23 mg EDTA/g E | |||
| methanol | AP | 1090.4 mg TE/g E | 3621.43 mg TE/g E | n.d | n.d | n.d | 58.08 mg TE/g E | n.d | 71.98 mg EDTA/g E | [39] | |
| F. thymifolia | methanol | AP | IC50 = 99 µg/mL | n.d | n.d | n.d | EC50 = 120 µg/mL | n.d | IC50 = 11 µg/mL | n.d | [23] |
| chloroform | IC50 = 120 µg/mL | n.d | n.d | n.d | EC50 = >1000 µg/mL | n.d | IC50 = >1000 µg/mL | n.d | |||
| ethyl acetate | AP | n.d | n.d | n.d | n.d | n.d | n.d | 71.66 ± 1.24% at 100 mg/mL | n.d | [21] | |
| n-butanol | n.d | n.d | n.d | n.d | n.d | n.d | 50.83 ± 1.65% at 100 mg/mL | n.d | |||
| chloroform | n.d | n.d | n.d | n.d | n.d | n.d | 49.91 ± 1.06% at 100 mg/mL | n.d | |||
| F. triandra | ethanol | AP | n.d | SC50 = 37.22 µg/mL | n.d | n.d | n.d | n.d | IC50 = 41.24 µg/mL | RC50 = 15.08 µg/mL | [34] |
| soxhlet | n.d | SC50 = 35.99 µg/mL | n.d | n.d | n.d | n.d | IC50 = 43.33 µg/mL | RC50 = 16.53 µg/mL | |||
3.2.2. Antimicrobial Activity
| Microbial Strain | Frankenia Species | Extract/ Fraction/EO | Organ | Assay | MIC (mg/mL) | MSI (%) | IZ (mm) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Gram+ bacteria | ||||||||
| Micrococcus luteus | F. laevis | ethanol | AP | microdilution | n.d | 83.16 ± 0.38 | n.d | [41] |
| F. laevis | EO | AP | disc diffusion | 0.5 | n.d | n.d | [43] | |
| Staphylococcus aureus | F. laevis | ethanol | AP | microdilution | n.d | 66.66 ± 1.25 | n.d | [41] |
| F. laevis | EO | AP | disc diffusion | 0.5 | n.d | n.d | [43] | |
| F. pulverulenta | EO | AP | well diffusion | n.d | n.d | - | [18] | |
| F. thymifolia | methanol chloroform | Sh Sh | disc diffusion disc diffusion | n.d n.d | n.d n.d | 8.6 8.0 | [23] | |
| F. thymifolia | n-butanol ethyl acetate | AP | disc diffusion disc diffusion | n.d n.d | n.d n.d | 9.0 11.0 | [42] | |
| Staphylococcus epidermidis | F. laevis | EO | AP | disc diffusion | 0.8 | n.d | n.d | [43] |
| F. hirsuta | ethanol | H | disc diffusion | n.d | n.d | 16.0 | [36] | |
| Enterococcus faecium | F. thymifolia | methanol chloroform | Sh Sh | disc diffusion disc diffusion | n.d n.d | n.d n.d | 9.5 8.5 | [23] |
| F. hirsuta | ethanol | H | disc diffusion | n.d | n.d | 16.0 | [36] | |
| Gram− bacteria | ||||||||
| Escherichia coli | F. laevis | ethanol | AP | microdilution | n.d | 56.18 ± 1.13 | n.d | [41] |
| F. pulverulenta | EO | AP | well diffusion | n.d | n.d | - | [18] | |
| F. laevis | EO | AP | disc diffusion | 0.8 | n.d | n.d | [43] | |
| F. thymifolia | methanol chloroform | Sh Sh | disc diffusion disc diffusion | n.d n.d | n.d n.d | 6.0 10.0 | [23] | |
| F. thymifolia | n-butanol ethyl acetate | AP | disc diffusion disc diffusion | n.d n.d | n.d n.d | 8.0 7.0 | [42] | |
| F. hirsuta | ethanol | H | disc diffusion | n.d | n.d | - | [36] | |
| Salmonella enterica ssp. arizonae | F. laevis | ethanol | AP | microdilution | n.d | 77.66 ± 0.14 | n.d | [41] |
| Salmonella typhimurium | F. laevis | EO | AP | disc diffusion | 0.5 | n.d | n.d | [43] |
| Salmonella typhi | F. thymifolia | methanol chloroform | Sh Sh | disc diffusion disc diffusion | n.d | n.d | 6.0 10.5 | [23] |
| Pseudomonas aeruginosa | F. laevis | EO | AP | disc diffusion | - | n.d | n.d | [43] |
| F. thymifolia | methanol chloroform | Sh Sh | disc diffusion disc diffusion | n.d n.d | n.d n.d | 6.0 8.1 | [23] | |
| F. thymifolia | n-butanol ethyl acetate | AP | disc diffusion disc diffusion | n.d n.d | n.d n.d | 12.0 7.0 | [42] | |
| F. hirsuta | ethanol | H | disc diffusion | n.d | n.d | 9.0 | [36] | |
| F. triandra | ethanol water | AP | microdilution macrodilution | 0.3 | n.d | n.d | [74] | |
| Klebsiella oxytoca | F. thymifolia | n-butanol ethyl acetate | AP | disc diffusion disc diffusion | n.d n.d | n.d n.d | 9.0 10.0 | [42] |
| Enterobacter aerogenes | F. hirsuta | ethanol | H | disc diffusion | n.d | n.d | - | [36] |
| Morganella morganii | F. triandra | ethanol-water | AP | microdilution macrodilution | 0.15 | n.d | n.d | [74] |
| Fungi | ||||||||
| Candida albicans | F. thymifolia | methanol chloroform | Sh Sh | disc diffusion disc diffusion | n.d n.d | n.d n.d | 6.0 9.5 | [23] |
| F. hirsuta | ethanol | H | disc diffusion | n.d | n.d | 12.0 | [36] | |
| Rhizoctonia solani | F. pulverulenta | EO | AP | well diffusion | n.d | n.d | 12.25 | [18] |
| Penicillium simplicissimum | F. pulverulenta | EO | AP | well diffusion | n.d | n.d | - | [18] |
| Fusarium oxysporum | F. pulverulenta | EO | AP | well diffusion | n.d | n.d | - | [18] |
| F. laevis | EO | AP | disc diffusion | - | n.d | n.d | [43] | |
| Penicillium citrinum | F. pulverulenta | EO | AP | well diffusion | n.d | n.d | - | [18] |
| Fusarium fujikuroi | F. pulverulenta | EO | AP | well diffusion | n.d | n.d | - | [18] |
| Aspergillus niger | F. laevis | EO | AP | disc diffusion | - | n.d | n.d | [43] |
| Alternaria sp. | F. laevis | EO | AP | disc diffusion | - | n.d | n.d | [43] |
| Penicillium sp. | F. laevis | EO | AP | disc diffusion | - | n.d | n.d | [43] |
| Parasite | ||||||||
| Acanthamoeba castellanii str. Neff. | F. thymifolia | n-butanol ethyl acetate | AP | modified Alamar Blue® | 95.43 66.25 | n.d n.d | n.d n.d | [42] |
| Leishmania amazonensis | F. thymifolia | n-butanol ethyl acetate | AP | modified Alamar Blue® | 100.13 99.36 | n.d n.d | n.d n.d | [42] |
| Leishmania donovani | F. thymifolia | n-butanol ethyl acetate | AP | modified Alamar Blue® | - - | n.d n.d | n.d n.d | [42] |
| Trypanosoma cruzi | F. thymifolia | n-butanol ethyl acetate | AP | modified Alamar Blue® | - - | n.d n.d | n.d n.d | [42] |
| Schistosoma mansoni | F. hirsuta | methanol | H | viability test | n.d | 46.80/68.50 | n.d | [31] |
| Virus | ||||||||
| HSV-1 | F. pulverulenta | acetone methanol | AP AP | neutral red incorporation | n.d n.d | n.d n.d | 489.5 486.2 | [75] |
3.2.3. Neuroprotective Activity
3.2.4. Tyrosinase Inhibition Activity
3.2.5. Anti-Inflammatory Activity
3.2.6. Carbonic Anhydrase II Inhibition Activity
3.2.7. Antidiabetic Activity
3.2.8. Anticancer Activity
3.2.9. Insecticidal Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/feature-stories/detail/traditional-medicine-has-a-long-history-of-contributing-to-conventional-medicine-and-continues-to-hold-promise (accessed on 19 September 2023).
- World Health Organization Report. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf (accessed on 19 September 2023).
- Fabricant, D.S.; Farnworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Kim, J.-W. Dynamics of zonal halophyte communities in salt marshes in the world. J. Mar. Isl. Cult. 2018, 7, 84–106. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.; Pinto, D.C.G.A.; Cunha, Â.; Silva, H. Halophytes as Medicinal Plants against Human Infectious Diseases. Appl. Sci. 2022, 12, 7493. [Google Scholar] [CrossRef]
- Arya, S.S.; Devi, S.; Ram, K.; Kumar, S.; Kumar, N.; Mann, A.; Kumar, A.; Chand, G. Halophytes: The Plants of Therapeutic Medicine. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019; pp. 271–287. [Google Scholar]
- Hernández-Ledesma, P.; Berendsohn, W.G.; Borsch, T.; Mering, S.V.; Akhani, H.; Arias, S.; Castañeda-Noa, I.; Eggli, U.; Eriksson, R.; Flores-Olvera, H.; et al. A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia 2015, 45, 281–383. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Ghahremani-nejad, F.; Zhang, D.-y.; Londo, J.P. A Systematic Overview of Frankeniaceae and Tamaricaceae from Nuclear rDNA and Plastid Sequence Data. Ann. Mo. Bot. Gard. 2004, 91, 401–409. [Google Scholar]
- Missouri Botanical Garden. Available online: https://www.mobot.org/mobot/research/apweb/orders/caryophyllalesweb.htm (accessed on 11 November 2023).
- Altameme, H. Systematics study of Frankenia L. (Frankeniaceae) in Iraq. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1232–1242. [Google Scholar]
- Rodrigues, M.J.; Jekő, J.; Cziáky, Z.; Pereira, C.G.; Custódio, L. The medicinal halophyte Frankenia laevis L.(sea heath) has in vitro antioxidant activity, α-glucosidase inhibition, and cytotoxicity towards hepatocarcinoma cells. Plants 2022, 11, 1353. [Google Scholar] [CrossRef]
- Hussein, S. Phenolic Sodium Sulfates of Frankenia laevis L. Pharmazie 2004, 59, 304–308. [Google Scholar] [CrossRef]
- Chegah, S.; Chehrazi, M.; Albaji, M. Effects of drought stress on growth and development Frankenia plant (Frankenia Leavis). Bulg. J. Agric. Sci. 2013, 19, 659–666. [Google Scholar]
- Malta Wild Plants. Available online: https://maltawildplants.com/FRNK/Frankenia_pulverulenta.php (accessed on 24 October 2023).
- Lopes, A.; Rodrigues, M.J.; Pereira, C.; Oliveira, M.; Barreira, L.; Varela, J.; Trampetti, F.; Custódio, L. Natural products from extreme marine environments: Searching for potential industrial uses within extremophile plants. Ind. Crops Prod. 2016, 94, 299–307. [Google Scholar] [CrossRef]
- Rehman, N.U.; Alsabahi, J.N.; Alam, T.; Rafiq, K.; Khan, A.; Hidayatullah; Khan, N.A.; Khan, A.L.; Al Ruqaishi, H.; Al-Harrasi, A. Chemical composition and biological activities of essential oil from aerial parts of Frankenia pulverulenta L. and Boerhavia elegans Choisy from Northern Oman. J. Essent. Oil-Bear. Plants 2021, 24, 1180–1191. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Wided, M.K.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. LC-MS identification and preparative HPLC isolation of Frankenia pulverulenta phenolics with antioxidant and neuroprotective capacities in PC12 cell line. Pharm. Biol. 2017, 55, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Boissier, P.E. Diagnoses Plantarum Orientalium Novarum; Series 2; (Reedition by Nabu Press: Charleston, SC, USA, 2012); Apud B. Herrmann: Lipsiae, Germany, 1854. [Google Scholar]
- Ben Mansour, R.; Ksouri, W.M.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. Assessment of Antioxidant Activity and Neuroprotective Capacity on PC12 Cell Line of Frankenia thymifolia and Related Phenolic LC-MS/MS Identification. Evid. Based Complement. Altern. Med. 2016, 2016, 2843463. [Google Scholar] [CrossRef] [PubMed]
- Harkat, H.; Haba, H.; Marcourt, L.; Long, C.; Benkhaled, M. An unusual lignan sulfate and aromatic compounds from Frankenia thymifolia Desf. Biochem. Syst. Ecol. 2007, 35, 176–179. [Google Scholar] [CrossRef]
- Megdiche-Ksouri, W.; Chaouachi, F.; M’Rabet, R.; Medini, F.; Zaouali, Y.; Trabelsi, N.; Ksoury, R.; Noumi, E.; Abdelly, C. Antioxidant and antimicrobial properties of Frankenia thymifolia Desf. fractions and their related biomolecules identification by gas chromatography/mass spectrometry (GC/MS) and high performance liquid chromatography (HPLC). J. Med. Plants Res. 2011, 5, 5754–5765. [Google Scholar]
- Altameme, H. A Chemical composition of Halophyte plant Frankenia pulverulenta L. (Frankeniaceae) in Iraq depending on GC-MS and FT-IR techniques. J. Chem. Pharm. Sci. 2017, 10, 26–33. [Google Scholar]
- Vahdati, N.; Tehranifar, A.; Kazemi, F. Assessing chilling and drought tolerance of different plant genera on extensive green roofs in an arid climate region in Iran. J. Environ. Manag. 2017, 192, 215–223. [Google Scholar] [CrossRef]
- Bueno, M.; Cordovilla, M.D. Spermidine Pretreatments Mitigate the Effects of Saline Stress by Improving Growth and Saline Excretion in Frankenia pulverulenta. Agronomy 2021, 11, 1515. [Google Scholar] [CrossRef]
- Harborne, J.B. Flavonoid bisulphates and their co-occurrences with ellagic acid in the Bixaceae, Frankeniaceae and related families. Phytochemistry 1975, 14, 1331–1337. [Google Scholar] [CrossRef]
- MaltaWildPlants. Available online: https://maltawildplants.com/FRNK/Frankenia_hirsuta.php (accessed on 24 October 2023).
- Vural, M.; Duman, H.; Aytac, Z.; Adigüzel, N. A new genus and three new species from Central Anatolia, Turkey. Turk. J. Bot. 2012, 36, 427–433. [Google Scholar] [CrossRef]
- Delitheos, A.; Tiligada, E.; Yannitsaros, A.; Bazos, I. Antiphage activity in extracts of plants growing in Greece. Phytomedecine 1997, 4, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Yousif, F.; Hifnawy, M.S.; Soliman, G.; Boulos, L.; Labib, T.; Mahmoud, S.; Ramzy, F.; Yousif, M.; Hassan, I.; Mahmoud, K.; et al. Large-scale in Vitro Screening of Egyptian Native and Cultivated plants for schistosomicidal activity. Pharm. Biol. 2007, 45, 501–510. [Google Scholar] [CrossRef]
- Altameme, H. Phytochemical analysis of Frankenia aucheri Jaub. et Spach (Frankeniaceae) by GC-MS and FT-IR techniques. Plant Arch. 2018, 18, 2263–2269. [Google Scholar]
- Abarsaji, G.A.; Mahdavi, M.; Jouri, M.H. Determination of Soil Salinity in Frankenia hirsuta L. Habitat (Case Study: Saline and Alkaline Rangelands of Golestan Province). J. Rangel. Sci. 2012, 2, 491–495. [Google Scholar]
- Torres Carro, R.; D’Almeida, R.E.; Isla, M.I.; Alberto, M.R. Antioxidant and anti-inflammatory activities of Frankenia triandra (J. Rémy) extracts. S. Afr. J. Bot. 2016, 104, 208–214. [Google Scholar] [CrossRef]
- Tan, Y.P.; Savchenko, A.I.; Broit, N.; Boyle, G.M.; Parsons, P.G.; Williams, C.M. The First plant 5,6-secosteroid from the australian arid zone species Frankenia foliosa. Eur. J. Org. Chem. 2017, 2017, 1498–1501. [Google Scholar] [CrossRef]
- Canli, K.; Simsek, O.; Yetgin, A.; Altuner, E.M. Determination of the chemical composition and antimicrobial activity of Frankenia hirsuta. Bangladesh J. Pharmacol. 2017, 12, 463–469. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Syed, Q.A.; Ishaq, A.; Al-Ghamdi, A.A.; Hatamleh, A.A. Botany, nutritional value; Phytochemical composition and biological activities of quinoa. Plants 2021, 10, 2258. [Google Scholar] [CrossRef]
- Woldemichael, G.M.; Wink, M. Identification and Biological Activities of Triterpenoid Saponins from Chenopodium quinoa. J. Agric. Food Chem. 2001, 49, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, R.; Megdiche-Ksouri, W.; Cluzet, S.; Krisa, S.; Mkadmini, K.; Richard, T.; Ksouri, R. Potential antioxidant capacities and neuroprotective properties of six Tunisian medicinal species. Int. J. Med. Plant Nat. Prod. 2017, 3, 45–54. [Google Scholar]
- Hussein, S.A.M. Flavonoid and methoxyellagic acid sodium sulphates from Frankenia laevis L. Pharmazie 2004, 59, 484–487. [Google Scholar] [PubMed]
- Jdey, A.; Falleh, H.; Ben Jannet, S.; Mkadmini Hammi, K.; Dauvergne, X.; Ksouri, R.; Magné, C. Phytochemical investigation and antioxidant, antibacterial and anti-tyrosinase performances of six medicinal halophytes. S. Afr. J. Bot. 2017, 112, 508–514. [Google Scholar] [CrossRef]
- Mennai, I.; Hanfer, M.; Esseid, C.; Benayache, S.; Ameddah, S.; Menad, A.; Benayache, F. Chemical composition, in vitro antiparasitic, antimicrobial and antioxidant activities of Frankenia thymifolia Desf. Nat. Prod. Res. 2020, 34, 3363–3368. [Google Scholar] [CrossRef] [PubMed]
- Saïdana, D.; Mahjoub, M.A.; Mighri, Z.; Chriaa, J.; Daamiand, M.; Helal, A.N. Studies of the essential oil composition, antibacterial and antifungal activity profiles of Frankenia laevis L. from Tunisia. J. Essent. Oil-Bear. Plants 2010, 22, 349–353. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Jaafar, H.Z.E.; Ghasemzadeh, A.; Ebrahimi, M. Fatty acid composition, antioxidant and antibacterial properties of the microwave aqueous extract of three varieties of Labisia pumila Benth. Biol. Res. 2015, 48, 9. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Flavier, M.E.; Rodriguez-Amaya, D.B.; Amaya-Farfán, J. Phytochemicals and functional foods. Current situation and prospect for developing countries. Segur. Aliment. Nutr. 2006, 13, 1–22. [Google Scholar] [CrossRef]
- Daoud, S.; Elbrik, K.; Tachbibi, N.; Bouqbis, L.; Brakez, M.; Harrouni, M.C. The potential use of halophytes for the development of marginal dry areas in Morocco. In Halophytes for Food Security in Dry Lands; Khan, M.A., Ozturk, M., Gul, B., Ahmed, M.Z., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 141–156. [Google Scholar]
- Alyemeni, M.N.; Sher, H.; Wijaya, L. Some observations on Saudi medicinal plants of veterinary importance. J. Med. Plants Res 2010, 4, 2298–2304. [Google Scholar]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and chelation in biochemistry and medicine: New approaches to controlling iron metabolism and treating related diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to Antibiotics: Are We in the Post-Antibiotic Era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Doenhoff, M.J.; Kusel, J.R.; Coles, G.C.; Cioli, D. Resistance of Schistosoma mansoni to praziquantel: Is there a problem? Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 465–469. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Protect. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Ozçelik, B.; Kusmenoglu, Ş.; Turkoz, S.; Abbasoglu, U. Antimicrobial activities of plants from the Apicaceae. Pharm. Biol. 2004, 42, 526–528. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Boussaada, O.; Ammar, S.; Saidana, D.; Chriaa, J.; Chraif, I.; Daami, M.; Helal, A.N.; Mighri, Z. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol. Res. 2008, 163, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotech. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Liu, J.-Y.; Hsui, Y.-R.; Chang, S.-T. Chemical polymorphism and antifungal activity of essential oils from leaves of different provenances of indigenous cinnamon (Cinnamomum osmophloeum). Bioresour. Technol. 2006, 97, 306–312. [Google Scholar] [CrossRef]
- Joshi, R.K. GC/MS analysis of the essential oil of Leucas indica from India. Nat. Prod. Commun. 2014, 9, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- El Hadidy, D.; El Sayed, A.M.; El Tantawy, M.; El Alfy, T. Phytochemical analysis and biological activities of essential oils of the leaves and flowers of Ageratum houstonianum Mill. cultivated in Egypt. J. Essent. Oil-Bear. Plants 2019, 22, 1241–1251. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharm. 2008, 116, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Seidel, V.; Taylor, P.W. In vitro activity of extracts and constituents of Pelagonium against rapidly growing mycobacteria. Int. J. Antimicrob. Agents 2004, 23, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.; Hall, A. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Badria, F.; Hetta, M.; Sarhan, R.M.; El-Din, M.E. Lethal effects of Helianthemum lippii (L.) on Acanthamoeba castellanii cysts in vitro. Korean J. Parasitol. 2014, 52, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Kirilmis, C.; Ahmedzade, M.; Servi, S.; Koca, M.; Kizirgil, A.; Kazaz, C. Synthesis and antimicrobial activity of some novel derivatives of benzofuran: Part 2. The synthesis and antimicrobial activity of some novel 1-(1-benzofuran-2-yl)-2-mesitylethanone derivatives. Eur. J. Med. Chem. 2008, 43, 300–308. [Google Scholar] [CrossRef]
- Bisignano, G.; Sanogo, R.; Marino, A.; Aquino, R.; D‘angelo, V.D.; Germano, M.P.; De Pasquale, R.; Pizza, C. Antimicrobial activity of Mitracarpus scaber extract and isolated constituents. Lett. Appl. Microbiol. 2000, 30, 105–108. [Google Scholar] [CrossRef]
- Jubie, S.; Ramesh, P.N.; Dhanabal, P.; Kalirajan, R.; Muruganantham, N.; Shanish Antony, A. Synthesis, antidepressant and antimicrobial activities of some novel stearic acid analogues. Eur. J. Med. Chem. 2012, 54, 931–935. [Google Scholar] [CrossRef]
- Canli, K.; Akata, I.; Altuner, E.M. In vitro antimicrobial activity screening of Xylaria hypoxylon. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 42–46. [Google Scholar] [CrossRef]
- Payne, D.J.; Warren, P.V.; Holmes, D.J.; Ji, Y.; Lonsdale, J.T. Bacterial fatty-acid biosynthesis: A genomics-driven target for antibacterial drug discovery. Drug Discov. Today 2001, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zampini, I.C.; Cuello, S.; Alberto, M.R.; Ordoñez, R.M.; Almeida, R.D.; Solorzano, E.; Isla, M.I. Antimicrobial activity of selected plant species from “the Argentine Puna” against sensitive and multi-resistant bacteria. J. Ethnopharm. 2009, 124, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ben Sassi, A.; Harzallah-Skhiri, F.; Bourgougnon, N.; Aouni, M. Antiviral activity of some Tunisian medicinal plants against Herpes simplex virus type 1. Nat. Prod. Res. 2008, 22, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.N.; Middleton, E.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Sig. Transduct. Target Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Rukmangadachar, L.A.; Bollu, P.C. Amyloid Beta Peptide; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Arias, C.; Montiel, T.; Quiroz-Báez, R.; Massieu, L. β-Amyloid neurotoxicity is exacerbated during glycolysis inhibition and mitochondrial impairment in the rat hippocampus in vivo and in isolated nerve terminals: Implications for Alzheimer’s disease. Exp. J. Neurol. 2002, 176, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Kumar, K.R.A.; Naveen, S. Phytochemicals Having Neuroprotective Properties from Dietary Sources and Medicinal Herbs. Pharmacogn. J. 2015, 7, 1–17. [Google Scholar] [CrossRef]
- Richard, T.; Poupard, P.; Nassra, M.; Papastamoulis, Y.; Iglésias, M.-L.; Krisa, S.; Waffo-Teguo, P.; Mérillon, J.-M.; Monti, J.-P. Protective effect of ε-viniferin on β-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg. Med. Chem. 2011, 19, 3152–3155. [Google Scholar] [CrossRef]
- Pamplona, S.; Sá, P.; Lopes, D.; Costa, E.; Yamada, E.; Silva, C.E.; Arruda, M.; Souza, J.; Da Silva, M. In Vitro Cytoprotective Effects and Antioxidant Capacity of Phenolic Compounds from the Leaves of Swietenia macrophylla. Molecules 2015, 20, 18777–18788. [Google Scholar] [CrossRef]
- Sylla, T.; Pouységu, L.; Da Costa, G.; Deffieux, D.; Monti, J.P.; Quideau, S. Gallotannins and tannic acid: First chemical syntheses and In vitro inhibitory activity on Alzheimer’s amyloid β-peptide aggregation. Angew. Chem. Int. Ed. 2015, 54, 8217–8221. [Google Scholar] [CrossRef]
- Zhu, J.T.T.; Choi, R.C.Y.; Chu, G.K.Y.; Cheung, A.W.H.; Gao, Q.T.; Li, J.; Jiang, Z.Y.; Dong, T.T.X.; Tsim, K.W.K. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: A comparison of different flavonoids in activating estrogenic effect and in preventing β-amyloid-induced cell death. J. Agric. Food Chem. 2007, 55, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.-W.; Wang, X.-M.; Ko, H.; Kwon, H.C.; Cha, J.W.; Yang, H.O. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid β-protein via the PI3K/Akt/Bad/BclXL-regulated mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2011, 672, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Richard, T.; Vitrac, X.; Mérillon, J.-M.; Valls, J.; Monti, J.-P. New polyphenols active on β-amyloid aggregation. Bioorg. Med. Chem. Lett. 2008, 18, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmology 2021, 190, 108353–108368. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.J.; Inestrosa, N.C. Interactions of AChE with Aβ aggregates in Alzheimer’s brain: Therapeutic relevance of IDN 5706. Front. Mol. Neurosci. 2011, 4, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents—Existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Neagu, E.; Paun, G.; Albu, C.; Radu, G.-L. Assessment of acetylcholinesterase and tyrosinase inhibitory and antioxidant activity of Alchemilla vulgaris and Filipendula ulmaria extracts. J. Taiwan Inst. Chem. Eng. 2015, 52, 1–6. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 2, S78–S87. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Torres Carro, R.; Isla, M.I.; Ríos, J.L.; Giner, R.M.; Alberto, M.R. Anti-inflammatory properties of hydroalcoholic extracts of Argentine Puna plants. Food Res. Int. 2015, 67, 230–237. [Google Scholar] [CrossRef]
- Rastogi, S.; Iqbal, M.S.; Ohri, D. In vitro study of anti-inflammatory and antioxidant activity of some medicinal plants and their interrelationship. Asian J. Pharm. Clin. Res. 2018, 11, 195–202. [Google Scholar] [CrossRef]
- Lindskog, S. Structure and Mechanism of Carbonic Anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Carta, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, M.; Halim, S.A.; Khan, Z.A.; Shafiq, Z.; Al-Harrasi, A. Quinazolinones as competitive inhibitors of carbonic anhydrase-II (human and bovine): Synthesis, in-vitro, in-silico, selectivity, and kinetics studies. Front. Chem. 2020, 8, 598095. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Gupta, V. Carbonic Anhydrase Inhibitors; StatPearls (Internet): Treasure Island, FL, USA, 2022. [Google Scholar]
- Supuran, C.T. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin. Ther. Pat. 2018, 28, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Custódio, L.; Lopes, A.; Oliveira, M.; Neng, N.R.; Nogueira, J.M.F.; Martins, A.; Rauter, A.P.; Varela, J.; Barreira, L. Unlocking the in vitro anti-inflammatory and antidiabetic potential of Polygonum maritimum. Pharm. Biol. 2017, 55, 1348–1357. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Nogueira, D.R.; Mitjans, M.; Infante, M.R.; Vinardell, M.P. Comparative sensitivity of tumor and non-tumor cell lines as a reliable approach for in vitro cytotoxicity screening of lysine-based surfactants with potential pharmaceutical applications. Int. J. Pharm. 2011, 420, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Taketomi, A.; Harimoto, N.; Tsujita, E.; Rikimaru, T.; Shirabe, K.; Shimada, M.; Maehara, Y. Antineoplastic effects of gamma linolenic acid on hepatocellular carcinoma cell lines. J. Clin. Biochemi. Nutr. 2010, 47, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Han, F.; Zhang, L.; Wang, L.; Kumar, M. Gamma linolic acid regulates PHD2 mediated hypoxia and mitochondrial apoptosis in DEN induced Hepatocellular carcinoma. Drug Des. Dev. Ther. 2018, 12, 4241–4252. [Google Scholar] [CrossRef] [PubMed]
- Gangadhar, K.N.; Rodrigues, M.J.; Pereira, H.; Gaspar, H.; Malcata, F.X.; Barreira, L.; Varela, J. Anti-hepatocellular carcinoma (HepG2) activities of monoterpene hydroxy lactones isolated from the marine microalga Tisochrysis lutea. Mar. Drugs 2020, 18, 567. [Google Scholar] [CrossRef] [PubMed]
- Vizetto-Duarte, C.; Custódio, L.; Gangadhar, K.N.; Lago, J.H.G.; Dias, C.; Matos, A.M.; Neng, N.; Nogueira, J.M.F.; Barreira, L.; Albericio, F.; et al. Isololiolide, a carotenoid metabolite isolated from the brown alga Cystoseira tamariscifolia, is cytotoxic and able to induce apoptosis in hepatocarcinoma cells through caspase-3 activation, decreased Bcl-2 levels, increased p53 expression and PARP cleavage. Phytomedicine 2016, 23, 550–557. [Google Scholar] [PubMed]
- Malek, S.N.A.; Shin, S.K.; Wahab, N.A.; Yaacob, H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules 2009, 14, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawy, S.; Hassan, A.Z.; Abdelhafez, M.A.; Mahmoud, K.; Mahrous, K.F.; Meselhy, M.R.; Sendker, J.; Abdel-Sattar, E. Cytotoxicity, genotoxicity, and gene expression changes induced by methanolic extract of Moringa stenopetala leaf with LC-qTOF-MS metabolic profile. Toxicon 2021, 203, 40–50. [Google Scholar] [CrossRef]
- Altiok, N.; Mezzadra, H.; Patel, P.; Koyuturk, M.; Altiok, S. A plant oxylipin, 12-oxo-phytodienoic acid, inhibits proliferation of human breast cancer cells by targeting cyclin D1. Breast Cancer Res. Treat. 2008, 109, 315–323. [Google Scholar] [CrossRef]
- Saidana, D.; Halima-Kamel, M.B.; Mahjoub, M.; Haouas, D.; Mighri, Z.; Helal, A. Insecticidal activities of Tunisian halophytic plant extracts against larvae and adults of Tribolium confusum. Tropicultura 2007, 25, 193–199. [Google Scholar]
- Phillipson, J.D. Natural products as drugs. Trans. R. Soc.Trop. Med. Hyg. 1994, 88, 17–19. [Google Scholar] [CrossRef]
- Verpoorte, R. Pharmacognosy in the new millennium: Leadfinding and Biotechnology. J. Pharm. Pharmacol. 2010, 52, 253–262. [Google Scholar] [CrossRef]











| Species Name | Synonym | Common Name | Distribution |
|---|---|---|---|
Frankenia laevis L. a  | Hypericopsis Boissier. [12] Frankenia canescens Presl. | Sea heath [13] | Portugal, Spain, France [13] Algeria, Morocco, Tunisia |
| Frankenia intermedia Costa. | Egypt [14], Iran [15] | ||
| Frankenia laevis Habl. ex Bieb. | |||
Frankenia pulverulenta L. b  | Frankenia nodiflora Lam. [16] | European sea heath [17] Annual sea heath [16] | Tunisia [18,19] |
Frankenia thymifolia Desf. c  | Frankenia reuteri Boiss. [20] | Thyme sea heath | North Africa [21,22,23] Oman [18], Iraq [24], Iran [25] |
| Spain [26], Portugal [17], England [27] | |||
Frankenia hirsuta L. d  | Frankenia aucheri Jaub. and Spach [12] Frankenia hirsuta L. var. erecta Boiss. | Millaih, Shuwaiwa [15] Hairy sea heath [28] | Saudi Arabia [18] |
| Frankenia salsuginea | Turkey [29] | ||
| Frankenia hispida D. C. | Greece [30], Egypt [31], Iraq [32], Iran [33] | ||
Frankenia triandra J. Rémy c  | Yareta Yaretilla [34] | Argentina, Chile Bolivia [34] | |
| Frankenia foliosa | Leafy sea heath | Australia [35] |
| Compound | Substituents | Species | References |
|---|---|---|---|
| (1) gallic acid | R1 = H; R2 = H; R3 = H; R4 = H | F. laevis, F. pulverulenta, F. thymifolia | [19,21,39,40,41] |
| (2) gallic acid-3-methyl ether | R1 = R2 = H; R3 = H; R4 = CH3 | F. laevis | [14,40] |
| (3) gallic acid-3-methyl ether-5-sodium sulphate | R1 = R3 = H; R2 = SO3Na; R4 = CH3 | “ a | [14] |
| (4) gallic acid sulfate | R1 = R2 = R3 = H; R4 = SO3H | “ | [13] |
| (5) methyl gallate-3,4-dimethyl ether | R1 = R3 = R4 = CH3; R2 = H | F. thymifolia | [42] |
| (6) 3-O-methylgallic acid-5-O-sulfate | R1 = R3 = H; R2 = CH3; R4 = SO3H | F. laevis | [13] |
| (7) 4-O-methylgallic acid | R1 = R2 = H; R3 = CH3; R4 = SO3H | “ | “ b |
| (8) trimethylgallate (eudesmic acid) | R1 = H; R2 = R3 = R4 = CH3 | F. hirsuta | [36] |
| (9) 4,5-dimethoxy-3-hydroxybenzoic acid methyl ester | R1 = R3 = R4 = CH3; R2 = H; | F. thymifolia | [22] |
| (10) salicylic acid | R1 = OH; R2 = R3 = R4 = H | “ | [23] |
| (11) p-hydroxybenzoic acid | R1 = R2 = R4 = H; R3 = OH | “ | [21] |
| (12) 2,5-dihydroxybenzoic acid | R1 = R4 = OH; R2 = R3 = H; | “ | [23] |
| (13) vanillic acid | R1 = R2 = H; R3 = OH; R4 = OCH3 | “ | [23] |
| (14) ellagic acid | R1 = R2 = R3 = R4 = H | F. laevis | [13,40] |
| (15) 3-O-methylellagic acid | R1 = CH3; R2 = R3 = R4 = H | “ | “ |
| (16) 3-O-methylellagic acid-4-O-sulfate | R1 = CH3; R2 = SO3H; R3 = R4 = H | “ | [13] |
| (17) 3,3′-di-O-methylellagic acid-4-O-sulfate | R1 = R4 = CH3; R2 = SO3H; R3 = H; | “ | “ |
| (18) 3,3′-di-O-methylellagic acid | R1 = R4 = CH3; R2 = R3 = H; | “ | [13,14] |
| (19) ellagic acid-3-methyl ether | R1 = CH3; R2 = R3 = R4 = H | “ | [14,40] |
| (20) ellagic acid-3-methyl ether-4′-sodium sulphate | R1 = R3 = H; R2 = SO3Na; R4 = CH3 | “ | [40] |
| (21) ellagic acid-3,3′-dimethyl ether-4-sodium sulphate | R1 = R4 = CH3; R2 = H; R3 = SO3Na; | “ | [14] |
| (22) ellagic acid-3,3′-dimethyl ether-4,4′-di-sodium sulphate | R1 = R4 = CH3; R2 = R3 = SO3Na | “ | “ |
| (23) ellagic acid-3-methyl ether-4-sodium sulphate | R1 = R2 = H; R3 = SO3Na; R4 = CH3 | “ | “ |
| (24) 3,3′,4-tri-O-methylellagic acid | R1 = R2 = R4 = CH3; R3 = H | “ | [13] |
| (25) 3,3′,4-tri-O-methylellagic acid-4′-O-sulfate | R1 = R2 = R4 = CH3; R3 = SO3H; | “ | “ |
| (26) 3-O-methylellagic acid-4′-O-glucoside | R1 = R3 = H; R2 = glucose; R4 = CH3 | “ | “ |
| (27) 3,3′-di-O-methylellagic acid-4-O-glucoside | R1 = CH3; R2 = glucose; R3 = H;R4 = CH3 | “ | “ |
| (28) E-cinnamic acid | R1 = H; R2 = H; R3 = H; R4 = H; R5 = OH | F. thymifolia | [23] |
| (29) E-2-hydroxycinnamic acid | R1 = R2 = R3 = H; R4 = R5 = OH | “ | “ |
| (30) caffeic acid | R1 = R2 = R5 = OH; R3 = R4 = H | “ | [39] |
| (31) chlorogenic acid | R1 = R2 = OH; R3 = R4 = H; R5 = 1,3,4-trihy droxycyclohexane-1-carboxylic acid | “ | [23,39] |
| (32) sinapic acid | R1 = OCH3; R2 = OH; R3 = OCH3; R4 = H; R5 = OH | “ | [39] |
| (33) caffeic acid sulfate | R1 = OSO3H; R2 = R5 = OH; R3 = R4 = H; | F. laevis | [13] |
| (34) p-coumaric acid | R1 = R3 = R4 = H; R2 = R5 = OH | “ | [41] |
| (35) p-coumaric acid 4-O-sulfate | R1 = R3 = R4 = H; R2 = OSO3H; R5 = OH | “ | [13] |
| (36) ferulic acid 4-O-sulfate | R1 = R3 = OCH3; R2 = OSO3H; R4 = H; R5 = OH | “ | “ |
| (37) coumaroyl hexose sulfate | R1 = R3 = R4 = H; R2 = OH; R5 = OCH2CHOSO3H(CHOH)3CH2OH | “ | “ |
| (38) caffeoyl pentose sulfate | R1 = OH; R2 = OH; R3 = H; R4 = H; R5 = OCH2CHOSO3H(CHOH)2CH2OH | “ | “ |
| (39) caffeoyl hexose sulfate | R1 = OH; R2 = OH; R3 = H; R4 = H; R5 = OCH2CHOSO3H(CHOH)3CH2OH | “ | “ |
| (40) feruloyl hexose sulfate | R1 = OCH3; R2 = OH; R3 = H; R4 = H; R5 = OCH2CHOSO3H(CHOH)3CH2OH | “ | “ |
| (41) N-cis-feruloyltyramine | R1 = R3 = R4 = H; R2 = OH; R5 = 4-(2-aminoethyl)phenol | “ | “ |
| (42) acetophenone-4-methylether | R = H | “ | [14] |
| (43) acetophenone-4-methylether-2-sodium sulfate | R = SO3Na | “ | [14] |
| (44) catechol | R1 = R2 = R5 = H; R3 = R4 = OH; | F. pulverulenta | [39] |
| (45) resorcinol | R1 = R3 = OH; R2 = R4 = R5 = H | “ | |
| (46) hydroxytyrosol | R1 = CH2CH2OH; R2 = R5 = H; R3 = R4 = OH; | F. thymifolia | [21] |
| (47) butenylpyrocatechol sulfate | R1 = OSO3H; R2 = OH; R3 = Butenyl; R4 = R5 = H | F. laevis | [13] |
| (48) butanoylpyrocatechol sulfate | R1 = OSO3H; R2 = OH; R3 = CO(CH2)2CH3; R4 = R5 = H | “ | “ |
| (49) eugenol | R1 = CH2CHCH2; R2 = R5 = H; R3 = OCH3; R4 = OH | “ | [43] |
| (50) 1,3-dithian-2-yl(phenyl)methanone | R1 = (1,3-dithian-2-yl)oxomethyl; R2 = R3 = R4 = R5 = H | F. hirsuta | [32] |
| (51) 3-tertbutyl-5-chloro-2-hydroxybenzo phenone | R1 = CO(phenyl); R2 = OH; R3 = C(CH3)3; R4 = H; R5 = Cl | F. pulverulenta | [24] |
| (52) posthumulone | F. thymifolia | [21] | |
| (53) α-tocopherol (vitamin E) | F. hirsuta | [36] |
| Compound | Substituents | Species | References |
|---|---|---|---|
| (54) kaempferol sulfate | R1 = SO3H; R2 = H | F. laevis | [13] |
| (55) kaempferol-3-sodium sulfate | R1 = SO3Na; R2 = H | “ a | [40] |
| (56) kaempferol-3-O-glucoside | R1 = glucosyl; R2 = H | F. thymifolia | [21] |
| (57) kaempferol-3-O-rutinoside | R1 = rutinosyl; R2 = H | F. pulverulenta | [39] |
| (58) kaempferol-7-sodium sulfate | R1 = H; R2 = SO3Na | F. laevis | [40] |
| (59) kaempferol-7-bisulfate | R1 = H; R2 = SO3H | F. pulverulenta | [27] |
| (60) kaempferol-3,7-disodium sulfate | R1 = SO3Na; R2 = SO3 Na | F. laevis | [40] |
| (61) kaempferol-7-bisulfate-3-glucuronide | R1 = glucuronide; R2 = SO3H | F. pulverulenta | [27] |
| (62) quercetin | R1 = H; R2 = H; R3 = H | F. thymifolia | [42] |
| (63) quercetin-3-O-methyl ether | R1 = H; R2 = CH3; R3 = H | “ | “ b |
| (64) quercetin-3-sodium sulfate | R1 = H; R2 = SO3Na; R3 = H | F. laevis | [40] |
| (65) quercetin-3-bisulfate | R1 = H; R2 = SO3H; R3 = H | F. pulverulenta | [27] |
| (66) quercetin-7-bisulfate | R1 = H; R2 = H; R3 = SO3H | “ | “ |
| (67) quercetin-7-sodium sulfate | R1 = R2 = H; R3 = (SO3)Na | F. laevis | [40] |
| (68) quercetin 7-bisulfate-3-glucuronide | R1 = H; R2 = glucuronide; R3 = SO3H | F. pulverulenta | [27] |
| (69) quercetin-3,7-disodium sulfate | R1 = H; R2 = SO3 Na; R3 = SO3Na | F. laevis | [40] |
| (70) quercetin-3′-bisulfate | R1 = (SO3H) Na; R2 = R3 = H | F. pulverulenta | [27] |
| (71) quercetin-3′-O-β-galactopyranoside | R1 = galactopyranosyl; R2 = R3 = H | F. thymifolia | [42] |
| (72) quercetin-3′-O-β-glucopyranoside | R1 = glucopyranosyl; R2 = R3 = H | “ | “ |
| (73) quercetin-3-O-galactoside (hyperoside) | R1 = R3 = H; R2 = galactosyl; | “ | [21] |
| (74) catechin | R1 = R2 = H | F. laevis, F. pulverulenta, F. thymifolia | [19,23,39,41] |
| (75) epigallocatechin | R1 = OH; R2 = H | F. pulverulenta | [39] |
| (76) epigallocatechino-3-gallate | R1 = OH; R2 = gallate | F. thymifolia | [23] |
| (77) prodelphinidin B-4 | “ | [21] | |
| (78) procyanidin (dimer 1,2 and 3) | F. pulverulenta | [19] | |
| (79) isorhamnetin-7-bisulfate | R1 = H; R2 = SO3H | “ | [27] |
| (80) isorhamnetin-7-bisulfate-3-glucuronide | R1 = glucuronide; R2 = SO3H | “ | “ |
| (81) isorhamnetin-O-pentosylhexoside | R1 = pentosyl-hexoside; R2 = H | F. laevis | [13] |
| (82) naringenin | F. thymifolia | [42] | |
| (83) luteolin-7-O-glucoside | F. pulverulenta, F. thymifolia | [21,39] |
| Compound | Substituents | Species | References |
|---|---|---|---|
| (84) lyoniresinol sulfate | F. laevis | [13] | |
| (85) lariciresinol | “ a | “ b | |
| (86) pinoresinol | R = H | F. thymifolia | [21] |
| (87) pinoresinol-4-sulfate | R = OSO3H | “ | [22] |
| (88) coumarin | F. pulverulenta | [39] |
| Compound | Substituents | Species | References |
|---|---|---|---|
| (89) S-methyl, N-(2-methyl-3-oxobutyl) dithiocarbamate | F. pulverulenta | [24] | |
| (90) 1,8-di-(4-nitrophenylmethyl)-3,6-diazahomoadamantan-9-one | “ a | “ b | |
| (91) pyrrolizin-1,7-dione-6-carboxylic acid, methyl ester | “ | “ | |
| (92) N-methyl, N-4-[1-(pyrrolidinyl)-2-butynyl] formamide | “ | “ | |
| (93) N-cyclooct-4-enyl acetamide | “ | “ | |
| (94) 2-(2-methyl-propenyl)-cyclohexanone oxime | “ | “ | |
| (95) 1-propyl-3,6-diaza homoadamantan-9-ol | R1 = H; R2 = (CH2)2CH3 | “ | “ |
| (96) 1,8-diethyl-3,6-diaza homoadamantan-9-ol | R1 = R2 = CH2CH3 | “ | “ |
| (97) 16,17-didehydrocuran | R1 = R2 = R3 = H | “ | “ |
| (98) (19S)-16,17-didehydrocuran-19,20-diol | R1 = H; R2 = R3 = OH | “ | “ |
| (99) 1-acetyl-20α-hydroxy-16-methylene strychnane | R1 = COCH3; R2 = OH; R3 = H | “ | “ |
| (100) dasycarpidan-1-methanol acetate | “ | “ | |
| (101) 2,7-diphenyl-1,6-dioxopyridazino [4,5:2′,3′]pyrrolo [4′,5′-d]pyridazine | “ | “ | |
| (102) dihydrotecomanine | F. pulverulenta, F. hirsuta | [24,32] | |
| (103) 2-acetylamino-3-hydroxypropionic acid | F. aucheri (irsute) | [32] | |
| (104) pterin-6-carboxylic acid | “ | “ | |
| (105) 18,19-didehydro-10-methoxycorynan-17-ol, acetate | “ | “ |
| Compound | Substituents | Species | References |
|---|---|---|---|
| (106) isololiolide | R = OH | F. laevis | [13] |
| (107) loliolide | R = OH | “ a | “ b |
| (108) dihydroactinidiolide | R = H | “ | “ |
| (109) α-pinene | F. pulverulenta | [18] | |
| (110) phytol | R = OH | F. laevis | [43] |
| (111) (E)-phytyl acetate | R = OCOCH3 | “ | “ |
| (112) isophytol | “ | “ | |
| (113) (E,E,E)-geranylgeraniol | F. pulverulenta | [18] | |
| (114) gibberellic acid | “ | [24] | |
| (115) caryophyllene oxide | F. laevis | [43] | |
| (116) (E)-nerolidol | “ | “ | |
| (117) (E,E)-farnesol | R = CH2OH | “ | “ |
| (118) (E,E)-farnesal | R = CHO | “ | “ |
| (119) (E,E)-farnesyl acetate | R = CH2OCOCH3 | “ | “ |
| (120) α-copaene-11-ol | F. pulverulenta | [18] | |
| (121) ledol | “ | “ | |
| (122) α-cadinol | “ | “ | |
| (123) tau-cadinol | “ | “ | |
| (124) torreyol | “ | “ | |
| (125) 6-epi-shyobunol | F. hirsuta | [32] | |
| (126) germacrene D | F. laevis | [43] | |
| (127) calarene | “ | “ | |
| (128) α-copaene | F. pulverulenta | [18] | |
| (129) β-humulene | “ | “ | |
| (130) α-selinene | “ | “ | |
| (131) β-selinene | “ | “ | |
| (132) ledene | “ | “ | |
| (133) δ-cadinene | “ | “ | |
| (134) γ-cadinene | F. laevis, F. pulverulenta | [18,43] | |
| (135) (E)-β-caryophyllene | “ | “ | |
| (136) allo-aromadendrene | “ | “ |
| Compound | Substituents | Species | References |
|---|---|---|---|
| (137) β-5,6-secosteroid | R = Et | F. foliosa | [35] |
| (138) 5-oxo-5,6-seco-3-cholesten-6-oic acid | R = H | “ a | “ b |
| (139) vitamin D (9,10-secosteroids) | “ | “ | |
| (140) brassinolide (6,7-secosteroids) | “ | “ | |
| (141) eringiacetal A | “ | “ | |
| (142) ethyl iso-allocholate | F. pulverulenta | [24] | |
| (143) 2-(3-acetoxy-4,4,14 trimethylandrost-8-en-17-yl)propanoic acid | “ | “ | |
| (144) γ-sitosterol | F. hirsuta | [36] |
| Compound | Substituents  | Species | References |
|---|---|---|---|
| (145) heptadecane | R1 = H; R2 = (CH2)14CH3 | F. laevis | [43] |
| (146) tricosane | R1 = H; R2 = (CH2)20CH3 | “ a | “ b |
| (147) tetracosane | R1 = H; R2 = (CH2)21CH3 | F. laevis, F. pulverulenta | [18,43] |
| (148) pentacosane | R1 = H; R2 = (CH2)22CH3 | “ | “ |
| (149) hexacosane | R1 = H; R2 = (CH2)23CH3 | F. laevis | [43] |
| (150) heptacosane | R1 = H; R2 = (CH2)24CH3 | “ | “ |
| (151) octacosane | R1 = H; R2 = (CH2)25CH3 | “ | “ |
| (152) nonacosane | R1 = H; R2 = (CH2)26CH3 | “ | “ |
| (153) triacontane | R1 = H; R2 = (CH2)27CH3 | “ | “ |
| (154) docosane | R1 = H; R2 = (CH2)19CH3 | F. pulverulenta | [18] |
| (155) n-heneicosane | R1 = H; R2 = (CH2)18CH3 | F. laevis | “ |
| (156) 2-methyloctacosane | R1 = CH3; R2 = (CH2)25CH3 | “ | “ |
| (157) hentriacontane | R1 = H; R2 = (CH2)28CH3 | “ | “ |
| (158) pentatriacontane | R1 = H; R2 = (CH2)32CH3 | “ | “ |
| (159) eicosane | R1 = H; R2 = (CH2)17CH3 | F. hirsuta | [36] |
| (160) 1-docosene | R1 = H; R2 = (CH2)18CHCH2 | F. laevis | [43] |
| (161) 1-nonade-cene | R1 = H; R2 = (CH2)15CHCH2 | F. hirsuta | [36] |
| Compound | Substituents  | Species | References |
|---|---|---|---|
| (162) caproic acid | R1 = (CH2)4CH3; R2 = H | F. laevis | [43] |
| (163) caprylic acid | R1 = (CH2)6CH3; R2 = H | F. hirsuta | [32] |
| (164) pelargonic acid | R1 = (CH2)7CH3; R2 = H | “ a | “ b |
| (165) lauric acid | R1 = (CH2)10CH3; R2 = H | F. laevis, F. thymifolia | [23,43] |
| (166) myristic acid | R1 = (CH2)12CH3; R2 = H | F. thymifolia | [23] |
| (167) palmitic acid | R1 = (CH2)14CH3; R2 = H | F. hirsuta, F. laevis, F. pulverulenta, F. thymifolia | [23,24,36,43] |
| (168) thapsic acid | R1 = (CH2)14COOH; R2 = H | F. laevis | [43] |
| (169) stearic acid | R1 = (CH2)16CH3; R2 = H | F. hirsuta, F. thymifolia | [23,32,36] |
| (170) behenic acid | R1 = (CH2)20CH3; R2 = H | “ | [23,36] |
| (171) lignoceric acid | R1 = (CH2)22CH3; R2 = H | F. hirsuta | [36] |
| (172) oleic acid | R1 = (Z)-heptadec-8-enyl; R2 = H | F. hirsuta, F. thymifolia | [23,36] |
| (173) elaïdic acid | R1 = (E)-heptadec-8-enyl; R2 = H | F. thymifolia | [23] |
| (174) linoleic acid | R1 = (8Z,11Z)-heptadeca-8,11-dienyl; R2 = H | F. hirsuta, F. thymifolia | [23,36] |
| (175) α-linolenic acid | R1 = (8Z,11Z,14Z)-heptadeca-8,11,14-trienyl; R2 = H | F. thymifolia | [23] |
| (176) gamolenic acid | R1 = (5Z,8Z,11Z)-hexadeca-5,8,11-trienyl; R2 = H | F. pulverulenta | [24] |
| (177) hydroxyoctadecadienoic acid | R1 = (1E,3E)-1-hydroxyheptadec-3-enylidene; R2 = H | F. laevis | [43] |
| (178) malyngic acid | R1 = (8S, 9E,11R,12R,13Z)-8,11,12-trihydroxyheptadeca-9,13-dienyl; R2 = H | “ | [13] |
| (179) methyl cis-12,13-epoxyoctadecanoate | R1 = cis-11,13-epoxyheptadecyl; R2 = CH3 | F. hirsuta | [32] |
| (180) methyl palmitate | R1 = pentadecyl; R2 = CH3 | F. laevis | [43] |
| (181) methyl linoleate | R1 = (8Z,11Z)-heptadeca-8,11-dienyl; R2 = CH3 | “ | “ |
| (182) methyl 12,15-octadecadiynoate | R1 = heptadeca-11,14-diynyl; R2 = CH3 | F. hirsuta | [32] |
| (183) methyl-11,13-dihydroxytetradec-5-ynoate | R1 = 10,11-dihydroxytridec-4-ynyl; R2 = CH3 | F. pulverulenta | [24] |
| Compound | Substituents | Species | References |
|---|---|---|---|
| (184) 5,7-dodecadiyn-1,12-diol | F. pulverulenta | [24] | |
| (185) hexadecan-1-ol | F. laevis | [43] | |
| (186) 3-O-methyl-d-glucose | F. pulverulenta, F. hirsuta | [24,32] | |
| (187) 6-acetyl-α-d-mannose | F. hirsuta | [32] | |
| (188) 6-acetyl-β-d-mannose | F. pulverulenta | [24] | |
| (189) α-d-glucopyranosyl-(1->3)-β-d-fructofuranosyl β-d-glucopyranoside | “ a | “ b | |
| (190) 4-O-(β-d-galactopyranosyl)-β-d-glucopyranose | “ | “ | |
| (191) desulphosinigrin | F. hirsuta | [32] | |
| (192) benzyl benzoate | R = phenyl | “ | “ |
| (193) benzyl cinnamate | R = styryl | “ | “ |
| (194) mesitylene | “ | [36] | |
| (195) 1,2,3,4,5,7-hexamethoxynaphthalene | F. thymifolia | [22] | |
| (196) pheophytin A | F. laevis | [13] | |
| (197) [(hexadecyloxy)methyl]oxirane | F. hirsuta | [32] | |
| (198) 8a-methyl-4H,5H-tetrahydropyrano[4,3-d]-1,3-dioxin | F. pulverulenta | [24] | |
| (199) 2-formyl-5-(hydroxymethyl)furan | F. hirsuta | [32] | |
| (200) 2-methyldecanal | F. laevis | [43] | |
| (201) (Z)-12-hydroxy-14-methyl-oxacyclotetradec-6-en-2-one | F. pulverulenta | [24] | |
| (202) 7-(1-hydroxypentyl)-2-oxabicyclo[3.3.0]oct-7-en-3-one | R = C4H9 | “ | “ |
| (203) citric acid | F. laevis | [13] | |
| (204) 12-oxophytodienoic acid | “ | “ | |
| (205) tuberonic acid sulfate | “ | “ | |
| (206) phthalic acid, butyl tetradecyl ester | R1 = nBu; R2 = tetradecyl | F. hirsuta | [32] |
| (207) phthalic acid, isobutyl octadecyl ester | R1 = iBu; R2 = octadecyl | “ | “ |
| (208) N,N’-bis(carbobenzyloxy)-l-lysinyl-l-valine methyl ester | “ | “ | |
| (209) 5-phenyloxymethyl-2-phenylhydrazino-4,5-dihydro-1,3-oxazole | “ | “ | |
| (210) 2,5-dihydroperoxy-2,5-dimethylhexane | “ | “ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaled, M.; Ouache, R.; Pale, P.; Harkat, H. Phytochemical Profiles and Biological Activities of Frankenia Species: A Review. Molecules 2024, 29, 980. https://doi.org/10.3390/molecules29050980
Khaled M, Ouache R, Pale P, Harkat H. Phytochemical Profiles and Biological Activities of Frankenia Species: A Review. Molecules. 2024; 29(5):980. https://doi.org/10.3390/molecules29050980
Chicago/Turabian StyleKhaled, Meyada, Rachid Ouache, Patrick Pale, and Hassina Harkat. 2024. "Phytochemical Profiles and Biological Activities of Frankenia Species: A Review" Molecules 29, no. 5: 980. https://doi.org/10.3390/molecules29050980
APA StyleKhaled, M., Ouache, R., Pale, P., & Harkat, H. (2024). Phytochemical Profiles and Biological Activities of Frankenia Species: A Review. Molecules, 29(5), 980. https://doi.org/10.3390/molecules29050980