Novel Meta-Diamide Compounds Containing Sulfide Derivatives Were Designed and Synthesized as Potential Pesticides
Abstract
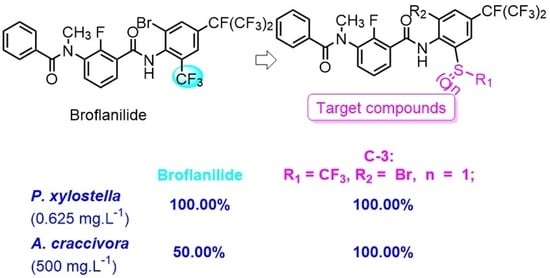
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structure-Activity Relationship
3. Materials and Methods
3.1. General Experimental Details
3.2. Synthesis and Characterization of the Compounds
3.2.1. General Procedure for the Preparation of Thioether-Containing m-Diamine Compound A and B
- 2-fluoro-3-(N-methylbenzamido)-N-(2-(methylthio)-4-(perfluoropropan-2-yl)phenyl)benzamide (A-1). Yellow solid (58%), m.p. 103–104 °C. 1H NMR (400 MHz, DMSO) δ 10.06 (s, 1H, -NH), 7.83 (d, J = 7.7 Hz, 1H, Ph-H), 7.67–7.61 (m, 1H, Ph-H), 7.60–7.55 (m, 2H, Ph-H), 7.53 (s, 1H, Ph-H), 7.42–7.22 (m, 6H, Ph-H), 3.35 (s, 3H, CH3), 2.50 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.6, 162.5, 138.4, 138.3, 136.1, 135.9, 133.4, 130.4, 129.8, 128.5, 128.1, 127.0, 125.4 (d, J = 4.3 Hz), 124.6, 124.2 (q, J = 272.3 Hz, CF3), 123.6, 123.4, 123.2, 122.0, 119.1, 39.3, 15.8. HRMS calcd for C25H18F8N2O2S [M + H]+ 563.1039, found 563.1035.
- 2-fluoro-3-(N-methylbenzamido)-N-(4-(perfluoropropan-2-yl)-2-((trifluoromethyl)thio)phenyl) benzamide (A-2). Yellow oil (66%). 1H NMR (400 MHz, DMSO) δ 10.63 (s, 1H, -NH), 8.07 (d, J = 8.5 Hz, 1H, Ph-H), 8.03–7.98 (m, 2H, Ph-H), 7.68–7.60 (m, 2H, Ph-H), 7.35–7.25 (m, 6H, Ph-H), 3.36 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.6, 162.7, 153.6, 143.9, 135.9, 134.1, 134.0, 133.8, 131.1, 130.4, 130.3, 130.2, 129.8, 128.4, 128.0, 126.6, 125.6, 125.5, 123.9, 123.0, 120.1, 39.6. HRMS calcd for C25H15F11N2O2S [M + H]+ 617.0757, found 617.0750.
- 2-fluoro-3-(N-methylbenzamido)-N-(4-(perfluoropropan-2-yl)-2-((2,2,2-trifluoroethyl)thio)phen--yl)benzamide (A-3). Yellow solid (58%), m.p. 119–120 °C. 1H NMR (400 MHz, DMSO) δ 10.24 (s, 1H, -NH), 8.03 (d, J = 8.6 Hz, 1H, Ph-H), 7.90 (d, J = 1.6 Hz, 1H, Ph-H), 7.72–7.66 (m, 2H, Ph-H), 7.61 (t, J = 5.9 Hz, 1H, Ph-H), 7.40–7.30 (m, 3H, Ph-H), 7.29–7.25 (m, 3H, Ph-H), 4.1 (q, J = 9.3 Hz, 2H, -CH2CF3), 3.35 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.6, 162.4, 153.7, 141.2, 135.9, 133.7, 133.3, 130.4, 130.3, 129.9, 128.5, 128.0, 127.7, 126.5, 126.0, 125.5, 125.4, 124.2, 122.9, 122.7, 122.0, 39.34, 35.6 (q, CH2, J = 31.3 Hz). HRMS calcd for C26H17F11N2O2S [M + H]+ 631.0913, found 631.0907.
- N-(2-bromo-6-(methylthio)-4-(perfluoropropan-2-yl)phenyl)-2-fluoro-3-(N-methylbenzamido) benzamide (B-1). Yellow solid (70%), m.p. 167–169 °C. 1H NMR (400 MHz, DMSO) δ 10.64 (s, 1H, -NH), 7.85 (d, J = 7.7 Hz, 1H, Ph-H), 7.66–7.60 (m, 3H, Ph-H), 7.54–7.51 (m, 1H, Ph-H), 7.30–7.24 (m, 5H, Ph-H), 3.35 (s, 3H, CH3), 2.79 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.7, 163.2, 140.4, 139.1, 135. 9, 134.6, 134.5, 133.2, 130.4, 129.2, 129.1, 128.4, 127.9, 126.9, 125.4, 125.3, 124.4, 123.2, 119.7, 113.4, 39.2, 14.24. HRMS calcd for C25H17BrF8N2O2S [M + H]+ 641.0145, found 641.0140.
- N-(2-bromo-4-(perfluoropropan-2-yl)-6-((trifluoromethyl)thio)phenyl)-2-fluoro-3-(N-methylben--zamido)benzamide (B-2). Yellow solid (82%), m.p. 85–86 °C. 1H NMR (400 MHz, DMSO) δ 10.71 (s, 1H, -NH), 8.29–8.06 (m, 2H, Ph-H), 7.85 (s, 1H, Ph-H), 7.61–7.58 (m, 2H, Ph-H), 7.48–7.40 (m, 5H, Ph-H), 3.33 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.6, 163.5, 155.9, 153.4, 138.9, 135.9, 133.3, 132.9, 131.8, 131.6, 130.5, 129.7, 129.4, 129.0, 128.5, 128.0, 126.8, 125.3, 124.9, 123.5, 122.3, 39.5. HRMS calcd for C25H14BrF11N2O2S [M + H]+ 694.9862, found 694.9854.
- N-(2-bromo-4-(perfluoropropan-2-yl)-6-((2,2,2-trifluoroethyl)thio)phenyl)-2-fluoro-3-(N-methyl--benzamido)benzamide (B-3). Yellow solid (70%), m.p. 142–144 °C. 1H NMR (400 MHz, DMSO) δ 10.53 (s, 1H, NH), 7.86 (s, 1H, Ph-H), 7.79 (s, 1H, Ph-H), 7.63–7.58 (m, 2H, Ph-H), 7.41–7.23 (m, 6H, Ph-H), 4.30–4.10 (m, 2H, -CH2CF3), 3.36 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.7, 162.6, 156.0, 153.5, 139.1, 138.1, 135.9, 133.2, 130.4, 129.2, 128.5, 128.0, 127.7, 126.5, 126.3, 125.6, 125.4, 124.9, 124.6, 124.4, 119.1, 39.5, 33.5 (q, CH2, J = 31.3 Hz). HRMS calcd for C26H17BrF11N2O2S [M + H]+ 709.0014, found 709.0018.
3.2.2. General Procedure for the Preparation of Sulfoxide-Containing m-Diamine Compound C
- 2-fluoro-3-(N-methylbenzamido)-N-(2-(methylsulfinyl)-4-(perfluoropropan-2-yl)phenyl)benzam-ide (C-1). White solid (62%), m.p. 112–113 °C. 1H NMR (400 MHz, DMSO) δ 10.87 (s, 1H, -NH), 8.08 (s, 1H, Ph-H), 7.95–7.90 (m, 2H, Ph-H), 7.63–7.58 (m, 2H, Ph-H), 7.38–7.24 (m, 6H, Ph-H), 3.35 (s, 3H, CH3), 2.83 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.6, 163.2, 155.9, 153.4, 141.8, 138.1, 135.9, 133.6, 130.4, 129.6, 128.5, 128.0, 127.5, 125.5, 124.3, 124.2, 124.1, 123.9, 122.4, 119.3, 42.3, 39.3. HRMS calcd for C25H18F8N2O3S [M + H]+ 579.0989, found 579.0986.
- N-(2-bromo-6-(methylsulfinyl)-4-(perfluoropropan-2-yl)phenyl)-2-fluoro-3-(N-methylbenzamido) benzamide (C-2). White solid (74%), m.p. 105–107 °C. 1H NMR (400 MHz, DMSO) δ 10.61 (s, 1H, -NH), 8.24 (s, 1H, Ph-H), 8.05 (s, 1H, Ph-H), 7.66 (t, J = 6.7 Hz, 1H, Ph-H), 7.60 (t, J = 6.3 Hz, 1H, Ph-H), 7.45–7.29 (m, 6H, Ph-H), 3.37 (s, 3H, CH3), 2.77 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.7, 163.2, 155.9, 149.9, 136.3, 135.9, 133.4, 133.2, 133.1, 132.4, 130.4, 129.2, 128.5, 128.0, 127.6, 126.1, 125.6, 125.5, 120.7, 119.1, 42.9, 39.3. HRMS calcd for C25H17BrF8N2O3S [M + H]+ 657.0094, found 657.0086.
- N-(2-bromo-4-(perfluoropropan-2-yl)-6-((trifluoromethyl)sulfinyl)phenyl)-2-fluoro-3-(N-methyl--benzamido)benzamide (C-3). Yellow solid(29%), m.p. 103–105 °C. 1H NMR (400 MHz, DMSO) δ 8.27 (s, 1H, Ph-H), 7.96 (s, 1H, Ph-H), 7.59–7.54 (m, 2H, Ph-H), 7.35–7.26 (m, 6H, Ph-H), 3.35 (s, 3H). 13C NMR (101 MHz, DMSO) δ 170.6, 163.0, 156.1, 152.8, 136.0, 134.8, 133.6, 132.4, 130.8, 130.3, 129.6, 129.5, 129.3, 128.4, 127.9, 125.1, 123.1, 122.2, 122.0, 119.2, 112.9, 39.3. HRMS calcd for C25H14BrF11N2O3S [M + H]+ 712.9795, found 712.9765.
- N-(2-bromo-4-(perfluoropropan-2-yl)-6-((2,2,2-trifluoroethyl)sulfinyl)phenyl)-2-fluoro-3-(N-methylbenzamido)benzamide (C-4). Yellow solid (30%), m.p. 80–82 °C. 1H NMR (400 MHz, DMSO) δ 10.66 (s, 1H, NH), 8.34 (s, 1H, Ph-H), 8.14 (s, 1H, Ph-H), 7.91 (s, 1H, Ph-H), 7.80–7.70 (m, 1H, Ph-H), 7.58–7.51 (m, 1H, Ph-H), 7.40–7.32 (m, 5H, Ph-H), 4.37 (dd, J = 22.5, 9.0 Hz, 1H, -CH2CF3), 4.04 (dd, J = 22.5, 9.0 Hz, 1H, -CH2CF3), 3.44 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.6, 166.6, 163.3, 153.6, 145.7, 136.4, 135.8, 133.8, 133.4, 133.2, 131.1, 130.4, 129.3, 128.4, 127.9, 127.8, 127.6, 126.3, 125.6, 123.2, 121.9, 57.5 (q, J = 26.2 Hz, -CH2CF3), 39.3. HRMS calcd for C26H17BrF11N2O3S [M + H]+ 724.9968, found 724.9963.
3.2.3. General Procedure for the Preparation of Sulfone-Containing m-Diamine Compound D
- 2-fluoro-3-(N-methylbenzamido)-N-(2-(methylsulfonyl)-4-(perfluoropropan-2-yl)phenyl)benza-mide (D-1). Yellow solid(60%), m.p. 87–89 °C. 1H NMR (400 MHz, DMSO) δ 10.40 (s, 1H, -NH), 8.61 (d, J = 9.4 Hz, 1H, Ph-H), 8.12 (s, 1H, Ph-H), 7.91 (s, 1H, Ph-H), 7.90–7.88 (m, 1H, Ph-H), 7.72–7.69 (m, 2H, Ph-H), 7.55–7.52 (m, 1H, Ph-H), 7.34–7.27 (m, 4H, Ph-H), 3.43 (s, 3H, CH3), 3.35 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.6, 166.6, 161.9, 139.7, 135.8, 134.5, 133.8, 133.4, 133.2, 132.6, 131.1, 130.3, 129.3, 128.5, 128.4, 127.9, 127.2, 126.0, 123.3, 121.7, 43.7, 39.3. HRMS calcd for C25H18F8N2O4S [M + H]+ 595.0938, found 595.0930.
- N-(2-bromo-6-(methylsulfonyl)-4-(perfluoropropan-2-yl)phenyl)-2-fluoro-3-(N-methylbenzamid--o)benzamide (D-2). White solid (58%), m.p. 207–209 °C. 1H NMR (400 MHz, DMSO) δ 10.70 (s, 1H, -NH), 8.46 (d, J = 1.9 Hz, 1H, Ph-H), 8.20 (d, J = 1.9 Hz, 1H, Ph-H), 7.66–7.63 (s, 2H, Ph-H), 7.40–7.28 (s, 6H, Ph-H), 3.38 (s, 3H, CH3), 3.37 (s, 3H, CH3). 13C NMR (101 MHz, DMSO) δ 170.8, 163.3, 155.9, 149.9, 136.4, 136.0, 133.5, 133.4, 132.5, 130.5, 129.3, 128.6, 128.1, 127.7, 126.2, 125.8, 125.7, 120.8, 120.7, 119.2, 43.1, 39.4. HRMS calcd for C25H17BrF8N2O4S [M + H]+ 673.0043, found 673.0036.
3.3. Insecticidal Activity Assay [17,18,19]
3.3.1. Rearing Conditions
3.3.2. Drug Preparation
3.3.3. Insecticidal Activity Methods
3.3.4. Data Statistics and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.; Zhang, J.; Gao, Y.; Hao, H.; Zhang, C.; Wang, F.; Zhang, L. Synthesis, acaricidal activity, and structure–activity relationships of novel phenyl trifluoroethyl thioether derivatives containing substituted benzyl groups. Pest Manag. Sci. 2024, 80, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Xu, L.; Li, Z.; Maienfisch, P. Design, Synthesis, and Properties of Silicon-Containing meta-Diamide Insecticides. J. Agric. Food Chem. 2023, 71, 18188–18196. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Bnaba, S. Broflanilide: A meta-diamide insecticide with a novel mode of action. Bioorg. Med. Chem. 2016, 24, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Lin, Y.; Zhang, Z.; Qiu, L.; Ding, W.; Gao, Q.; Xue, J.; Li, Y.; He, H. The transcriptomic profile of Spodoptera frugiperda differs in response to a novel insecticide, cyproflanilide, compared to chlorantraniliprole and avermectin. BMC Genomics 2023, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Wu, D.; Wang, J.; Liu, J.; Zhou, L.; Liu, M. Design, synthesis, and insecticidal activities of novel meta-diamide compounds containing sulfide, sulfoxide and sulfone. Tetrahedron Lett. 2023, 118, 154388. [Google Scholar] [CrossRef]
- Devendar, P.; Yang, G.F. Sulfur-Containing Agrochemicals. Top. Curr. Chem. 2017, 375, 82–126. [Google Scholar] [CrossRef] [PubMed]
- Kurmanbayeva, M.; Sekerova, T.; Tileubayeva, Z.; Kaiyrbekov, T.; Kusmangazinov, A.; Shapalov, S.; Madenova, A.; Burkitbayev, M.; Bachilova, N. Influence of new sulfur-containing fertilizers on performance of wheat yield. Saudi J. Biol. Sci. 2021, 28, 4644–4655. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sharma, R.K.; Battu, R.S.; Singh, B. Dissipation kinetics of Flubendiamide on chili and soil. Bull. Environ. Contam. Toxicol. 2009, 83, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Mahler, B.J.; Van Metre, P.C.; Wilson, J.T.; Musgrove, M.; Zaugg, S.D.; Burkhardt, M.R. Fipronil and its degradates in indoor and outdoor dust. Environ. Sci. Technol. 2009, 43, 5665–5670. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Mukawa, S. Characteristics biological activities of the novel acaricide flupentiofenox against phytophagous mites. J. Pestic. Sci. 2023, 48, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Ban, L.; Li, P.; Li, K.; Xu, J. Biphenyl Compounds and Their Preparation, Agricultural Compositions and Use in the Control of Mites. CN 105541682, 4 May 2016. [Google Scholar]
- Wang, J.; Xiang, J.; Wu, M.; Long, H.; Wu, D.; Liu, J. Design, synthesis, and insecticidal activities of novel meta-diamide compounds containing ethyl acetate and their derivatives. J. Chem. Biodivers. 2023, 20, e202300060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Li, H.; Shang, J.F.; Li, Z.M.; Wang, B.L. Synthesis and insecticidal evaluation of novel sulfide-containing amide derivatives as potential ryanodine receptor modulators. Chin. Chem. Lett. 2022, 33, 501–507. [Google Scholar] [CrossRef]
- Mueller, P.; Vriza, A.; Clayton, A.D.; May, O.S.; Govan, N.; Notman, S.; Ley, S.V.; Chamberlain, T.W.; Bourne, R.A. Exploring the chemical space of phenyl sulfide oxidation by automated optimization. React. Chem. Eng. 2023, 8, 538–542. [Google Scholar] [CrossRef]
- Wang, J.; Qin, S.; Sheng, Z.; Zhang, J.; Zhang, L. Synthesis and insecticidal activity of Broflanilide. Xiandai Nongyao 2020, 19, 25–29. [Google Scholar]
- Luo, C.; Ma, W.; Lv, L.; Pang, H.; Xiang, J.; Zhou, L.; Yin, D.; Liu, J. Synthesis and insecticidal activity of novel meta-diamide compounds containing cyclopropyl group. Chin. J. Org. Chem. 2020, 40, 2963–2970. [Google Scholar] [CrossRef]
- Hu, Z.; Feng, X.; Lin, Q.; Chen, H.; Li, Z.; Yin, F.; Liang, P.; Gao, X. Biochemical mechanism of Chlorantraniliprole resistance in the Diamondback moth, Plutella xylostella Linnaeus. J. Integr. Agric. 2014, 13, 2452–2459. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.; Choi, M.Y.; Paik, C.K.; Seo, H.Y.; Kalaivani, K. Toxicity and physiological effects of neem pesticides applied to rice on the Nilaparvata lugens Stal, the brown planthopper. Ecotox. Environ. Safe 2009, 72, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Zhu, B.; Yuan, J.; Yu, J.; Dong, D.; Zhou, D.; Hu, D.; Chen, J. Bioactivity and field efficacy of novel insecticide against different lepidopterous pests. Chin. J. Pestic. Sci. 2013, 15, 159–164. [Google Scholar]




| Entry | Solvent | Reaction Condition (Equiv. of Reactant) | Temp. (°C) | Yield of 6a (%) a |
|---|---|---|---|---|
| 1 | DMF | 5:NaOH:FeSO4·7H2O:(CF3)2FCI 1:8.5:1.5:1.5 | rt | nr |
| 2 | reflux | nr | ||
| 3 | 5:NaOH:FeSO4·7H2O:(CF3)2FCI 1:9.5:1.5:1.5 | reflux | nr | |
| 4 | 5:NaOH:FeSO4·7H2O:(CF3)2FCI 1:9.5:3:3 | reflux | nr | |
| 5 | EA-H2O | 5:Na2S2O4:NaHCO3:IPC-TBA-HS:(CF3)2FCI 1:1.1:1.1:0.1:1.1 | rt | ur |
| 6 | 40 | ur | ||
| 7 | reflux | ur | ||
| 8 | 5:Na2S2O4:NaHCO3:IPC-TBA-HS:(CF3)2FCI 1:1.1:1.1:0.3:1.1 | reflux | ur | |
| 9 | 5:Na2S2O4:NaHCO3:IPC-TBA-HS:(CF3)2FCI 1:1.1:1.1:0.3:2 | reflux | 42% | |
| 10 | ||||
| 11 |
| Entry | Solvent | Reaction Condition (Equiv. of Reactant) | Temp. (°C) | Yield of C-3 (%) a |
|---|---|---|---|---|
| 1 | 1,4-dioxane | B-2:m-CPBA:K2CO3 1:3:1 | −10 | nr |
| 2 | rt | nr | ||
| 3 | reflux | nr | ||
| 4 | B-2:m-CPBA:K2CO3 1:4:1 | reflux | nr | |
| 5 | B-2:m-CPBA:K2CO3 1:5:1 | reflux | nr | |
| 6 | CF3COOH | B-2:H2O2 1:1 | 0 | nr |
| 7 | rt | ur | ||
| 8 | reflux | ur | ||
| B-2:H2O2 1:3 | −10 | dm | ||
| 9 | 0 | dm | ||
| 10 | rt | dm | ||
| 11 | reflux | dm | ||
| 12 | B-2:H2O2 1:5 | −10 | 29% | |
| 13 | rt | dm |
| No. | Lethal Rate (%) at a Concentration (mg·L−1) of P. xylostella | |||||||
|---|---|---|---|---|---|---|---|---|
| 500 | 200 | 50 | 25 | 12.5 | 10 | 2.5 | 0.625 | |
| A-1 | 100.00 | 100.00 | 0.00 | - | - | - | - | - |
| A-2 | 100.00 | 86.67 | - | - | - | - | - | - |
| A-3 | 83.33 | - | - | - | - | - | - | - |
| B-1 | 100.00 | 100.00 | 30.00 | - | - | - | - | - |
| B-2 | 10.00 | 100.00 | 100.00 | 0.00 | - | - | - | - |
| B-3 | 100.00 | 0.00 | - | - | - | - | - | - |
| C-1 | 100.00 | 100.00 | 20.00 | - | - | - | - | - |
| C-2 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 60.00 |
| C-3 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| C-4 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 90.00 | 100.00 |
| D-1 | 100.00 | 56.67 | - | - | - | - | - | - |
| D-2 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 73.33 |
| Broflanilide | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Dinotefuran | - | - | - | - | - | - | - | - |
| Blank control | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| No. | Lethal Rate (%) at a Concentration of (mg·L−1) | ||||
|---|---|---|---|---|---|
| A. craccivora | N. lugens | ||||
| 500 | 200 | 50 | 12.5 | 500 | |
| A-1 | 36.36 | - | - | - | 4.76 |
| A-2 | 31.18 | - | - | - | 0.00 |
| A-3 | 13.79 | - | - | - | 0.00 |
| B-1 | - | - | - | - | 0.00 |
| B-2 | 100.00 | 29.11 | 29.47 | 13.10 | 4.55 |
| B-3 | - | - | - | - | - |
| C-1 | 12.50 | - | - | - | 0.00 |
| C-2 | 100.00 | 15.48 | 18.57 | 10.53 | 0.00 |
| C-3 | 100.00 | 75.00 | 59.09 | 9.09 | 0.00 |
| C-4 | 33.90 | - | - | - | 0.00 |
| D-1 | 0.00 | - | - | - | 0.00 |
| D-2 | 100.00 | 66.67 | 48.61 | 31.1 | 0.00 |
| Broflanilide | 50.00 | - | - | - | 0.00 |
| Dinotefuran | 100.00 | 100.00 | 73.53 | 48.94 | 100.00 |
| Blank control | 0.00 | 0.00 | 0.00 | 0.00 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Dang, S.; Zhang, Y.; Zhou, S. Novel Meta-Diamide Compounds Containing Sulfide Derivatives Were Designed and Synthesized as Potential Pesticides. Molecules 2024, 29, 1337. https://doi.org/10.3390/molecules29061337
Wu J, Dang S, Zhang Y, Zhou S. Novel Meta-Diamide Compounds Containing Sulfide Derivatives Were Designed and Synthesized as Potential Pesticides. Molecules. 2024; 29(6):1337. https://doi.org/10.3390/molecules29061337
Chicago/Turabian StyleWu, Jingwen, Shuaihui Dang, Yan Zhang, and Sha Zhou. 2024. "Novel Meta-Diamide Compounds Containing Sulfide Derivatives Were Designed and Synthesized as Potential Pesticides" Molecules 29, no. 6: 1337. https://doi.org/10.3390/molecules29061337
APA StyleWu, J., Dang, S., Zhang, Y., & Zhou, S. (2024). Novel Meta-Diamide Compounds Containing Sulfide Derivatives Were Designed and Synthesized as Potential Pesticides. Molecules, 29(6), 1337. https://doi.org/10.3390/molecules29061337